The Necdin-Wnt pathway causes epigenetic peroxisome proliferator-activated receptor gamma repression in hepatic stellate cells
- PMID: 20663865
- PMCID: PMC2945539
- DOI: 10.1074/jbc.M110.156703
The Necdin-Wnt pathway causes epigenetic peroxisome proliferator-activated receptor gamma repression in hepatic stellate cells
Abstract
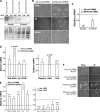
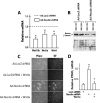
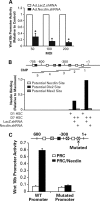
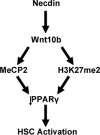
Hepatic stellate cells (HSCs), vitamin A-storing liver pericytes, undergo myofibroblastic trans-differentiation or "activation" to participate in liver wound healing. This cellular process involves loss of regulation by adipogenic transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ). Necdin, a melanoma antigen family protein, promotes neuronal and myogenic differentiation while inhibiting adipogenesis. The present study demonstrates that necdin is selectively expressed in HSCs among different liver cell types and induced during their activation in vitro and in vivo. Silencing of necdin with adenovirally expressed shRNA, reverses activated HSCs to quiescent cells in a manner dependent on PPARγ and suppressed canonical Wnt signaling. Promoter analysis, site-directed mutagenesis, and chromatin immunoprecipitation demonstrate that Wnt10b, a canonical Wnt induced in activated HSCs, is a direct target of necdin. Necdin silencing abrogates three epigenetic signatures implicated in repression of PPARγ: increased MeCP2 (methyl CpG binding protein 2) and HP-1α co-repressor recruitments to Pparγ promoter and enhanced H3K27 dimethylation at the exon 5 locus, again in a manner dependent on suppressed canonical Wnt. These epigenetic effects are reproduced by antagonism of canonical Wnt signaling with Dikkopf-1. Our results demonstrate a novel necdin-Wnt pathway, which serves to mediate antiadipogenic HSC trans-differentiation via epigenetic repression of PPARγ.
Figures
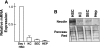

Similar articles
-
Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration.J Biol Chem. 2012 Mar 23;287(13):10355-10367. doi: 10.1074/jbc.M111.312751. Epub 2012 Feb 1. J Biol Chem. 2012. PMID: 22298767 Free PMC article.
-
Wnt signaling in liver fibrosis: progress, challenges and potential directions.Biochimie. 2013 Dec;95(12):2326-35. doi: 10.1016/j.biochi.2013.09.003. Epub 2013 Sep 13. Biochimie. 2013. PMID: 24036368 Review.
-
Morphogens and hepatic stellate cell fate regulation in chronic liver disease.J Gastroenterol Hepatol. 2012 Mar;27 Suppl 2(Suppl 2):94-8. doi: 10.1111/j.1440-1746.2011.07022.x. J Gastroenterol Hepatol. 2012. PMID: 22320925 Free PMC article. Review.
-
Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor γ in hepatic stellate cells for their antifibrotic effect.Hepatology. 2012 Apr;55(4):1271-81. doi: 10.1002/hep.24792. Epub 2012 Mar 1. Hepatology. 2012. PMID: 22095555 Free PMC article.
-
Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis.Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G39-49. doi: 10.1152/ajpgi.00263.2007. Epub 2007 Nov 15. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18006602
Cited by
-
Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration.J Biol Chem. 2012 Mar 23;287(13):10355-10367. doi: 10.1074/jbc.M111.312751. Epub 2012 Feb 1. J Biol Chem. 2012. PMID: 22298767 Free PMC article.
-
Metformin-mediated Bambi expression in hepatic stellate cells induces prosurvival Wnt/β-catenin signaling.Cancer Prev Res (Phila). 2012 Apr;5(4):553-61. doi: 10.1158/1940-6207.CAPR-12-0053. Epub 2012 Mar 9. Cancer Prev Res (Phila). 2012. PMID: 22406377 Free PMC article.
-
Necdin controls proliferation of white adipocyte progenitor cells.PLoS One. 2012;7(1):e30948. doi: 10.1371/journal.pone.0030948. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292082 Free PMC article.
-
Morphogen-related therapeutic targets for liver fibrosis.Clin Res Hepatol Gastroenterol. 2015 Sep;39 Suppl 1(0 1):S69-74. doi: 10.1016/j.clinre.2015.05.017. Epub 2015 Jul 20. Clin Res Hepatol Gastroenterol. 2015. PMID: 26206577 Free PMC article. Review.
-
β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis.Gastroenterology. 2015 Jun;148(7):1294-310. doi: 10.1053/j.gastro.2015.02.056. Epub 2015 Mar 5. Gastroenterology. 2015. PMID: 25747274 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

