Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells
- PMID: 20660297
- PMCID: PMC2940472
- DOI: 10.1210/me.2010-0125
Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells
Abstract
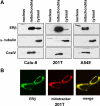
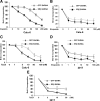
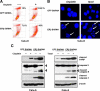
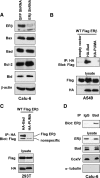
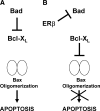
Recent studies have demonstrated the presence of estrogen receptor (ER)beta in the mitochondria in various cell types and tissues, but the exact function of this localization remains unclear. In this study, we have examined the function of mitochondrial ERbeta in non-small-cell lung cancer (NSCLC) cells. Down-regulation of ERbeta by short hairpin RNA constructs sensitized NSCLC cells to various apoptosis-inducing agents such as cisplatin, taxol, and etoposide. The increased growth inhibition and induction of apoptosis in ERbeta-knockdown cells was observed irrespective of estrogen treatment, suggesting a ligand-independent role of ERbeta in regulating the intrinsic apoptotic pathway. Further, ERbeta from the mitochondrial fraction physically interacted with the proapoptotic protein Bad, in a ligand-independent manner. Glutathione-S-transferase pull-down assays and molecular modeling studies revealed that the DNA-binding domain and hinge region of ERbeta, and the BH3 domain of Bad were involved in these interactions. Further investigations revealed that ERbeta inhibited Bad function by disrupting Bad-Bcl-X(L) and Bad-Bcl-2 interactions. Reintroduction of ERbeta in the mitochondria of ERbeta knockdown cells reversed their sensitivity to cisplatin. Overall, our results demonstrate a ligand-independent role of ERbeta in regulating apoptosis, revealing a novel function for ERbeta in the mitochondria.
Figures


Similar articles
-
[Mitochondrial estrogen receptor β inhibits non-small cell lung cancer cell apoptosis via interaction with Bad].Nan Fang Yi Ke Da Xue Xue Bao. 2015 Jan;35(1):98-102. Nan Fang Yi Ke Da Xue Xue Bao. 2015. PMID: 25613618 Chinese.
-
Mitochondrial estrogen receptor β inhibits cell apoptosis via interaction with Bad in a ligand-independent manner.Mol Cell Biochem. 2015 Mar;401(1-2):71-86. doi: 10.1007/s11010-014-2293-y. Epub 2014 Dec 19. Mol Cell Biochem. 2015. PMID: 25524600
-
Testicular orphan nuclear receptor 4-associated protein 16 promotes non-small cell lung carcinoma by activating estrogen receptor β and blocking testicular orphan nuclear receptor 2.Oncol Rep. 2013 Jan;29(1):297-305. doi: 10.3892/or.2012.2107. Epub 2012 Oct 26. Oncol Rep. 2013. PMID: 23129017 Free PMC article.
-
Estrogen receptor-β in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis.Ann N Y Acad Sci. 2015 Sep;1350:52-60. doi: 10.1111/nyas.12872. Epub 2015 Aug 24. Ann N Y Acad Sci. 2015. PMID: 26301952 Review.
-
Estrogen receptors, antiestrogens, and non-small cell lung cancer.Biochemistry (Mosc). 2010 Dec;75(12):1421-7. doi: 10.1134/s0006297910120011. Biochemistry (Mosc). 2010. PMID: 21314611 Review.
Cited by
-
Sterile α Motif Domain Containing 9 Is a Novel Cellular Interacting Partner to Low-Risk Type Human Papillomavirus E6 Proteins.PLoS One. 2016 Feb 22;11(2):e0149859. doi: 10.1371/journal.pone.0149859. eCollection 2016. PLoS One. 2016. PMID: 26901061 Free PMC article.
-
Estrogen, Estrogen Receptor and Lung Cancer.Int J Mol Sci. 2017 Aug 5;18(8):1713. doi: 10.3390/ijms18081713. Int J Mol Sci. 2017. PMID: 28783064 Free PMC article. Review.
-
[Recent Advances in Association of Estrogen and Non-small Cell Lung Cancer].Zhongguo Fei Ai Za Zhi. 2017 Jul 20;20(7):499-504. doi: 10.3779/j.issn.1009-3419.2017.07.09. Zhongguo Fei Ai Za Zhi. 2017. PMID: 28738967 Free PMC article. Review. Chinese.
-
Estrogen receptors promote NSCLC progression by modulating the membrane receptor signaling network: a systems biology perspective.J Transl Med. 2019 Sep 11;17(1):308. doi: 10.1186/s12967-019-2056-3. J Transl Med. 2019. PMID: 31511014 Free PMC article.
-
Upregulation of estrogen receptor beta protein but not mRNA predicts poor prognosis and may be associated with enhanced translation in non-small cell lung cancer: a systematic review and meta-analysis.J Thorac Dis. 2021 Jul;13(7):4281-4300. doi: 10.21037/jtd-21-658. J Thorac Dis. 2021. PMID: 34422356 Free PMC article.
References
-
- Ascenzi P, Bocedi A, Marino M 2006 Structure-function relationship of estrogen receptor α and β: impact on human health. Mol Aspects Med 27:299–402 - PubMed
-
- Matthews J, Gustafsson JA 2003 Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv 3:281–292 - PubMed
-
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 - PubMed
-
- Cowley SM, Parker MG 1999 A comparison of transcriptional activation by ERα and ERβ. J Steroid Biochem Mol Biol 69:165–175 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

