Asymmetrically inherited multidrug resistance transporters are recessive determinants in cellular replicative ageing
- PMID: 20657593
- PMCID: PMC2917193
- DOI: 10.1038/ncb2085
Asymmetrically inherited multidrug resistance transporters are recessive determinants in cellular replicative ageing
Abstract
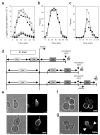
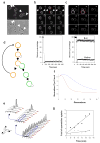
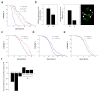
Cellular ageing is known to correlate with the accumulation of many harmful agents, but it is unclear whether ageing can also result from the deterioration of components that are beneficial to the cell, but have a low rate of renewal. Here, we report a group of plasma membrane-associated transporters in yeast, belonging to the multidrug resistance (MDR) protein families, that may represent the latter type of ageing determinants. During the division of a yeast cell, newly synthesized transporter proteins are deposited mostly into the growing bud, whereas previously synthesized MDR proteins remain tightly associated with the mother cortex. Thus, the new and old pools of membrane-bound MDR proteins are spatially segregated during yeast asymmetric cell division, with the older pool stably inherited by the ageing mother. A model based on the observed dynamics of MDR protein inheritance and turnover predicted a decline in MDR activity as the mother cell advances in replicative age. As MDR proteins have crucial roles in cellular metabolism, detoxification and stress response, their collective decline may lead to fitness loss at an advanced age. Supporting this hypothesis, mutants lacking certain MDR genes exhibited a reduced replicative lifespan (RLS), whereas introduction of only one extra copy of these MDR genes extended RLS.
Conflict of interest statement
Figures

Similar articles
-
Sphingolipids facilitate age asymmetry of membrane proteins in dividing yeast cells.Mol Biol Cell. 2017 Oct 1;28(20):2712-2722. doi: 10.1091/mbc.E17-05-0335. Epub 2017 Aug 2. Mol Biol Cell. 2017. PMID: 28768828 Free PMC article.
-
Heterologous expression of a Tpo1 homolog from Arabidopsis thaliana confers resistance to the herbicide 2,4-D and other chemical stresses in yeast.Appl Microbiol Biotechnol. 2009 Oct;84(5):927-36. doi: 10.1007/s00253-009-2025-5. Epub 2009 May 14. Appl Microbiol Biotechnol. 2009. PMID: 19440702
-
The multidrug resistance transporters of the major facilitator superfamily, 6 years after disclosure of Saccharomyces cerevisiae genome sequence.J Biotechnol. 2002 Sep 25;98(2-3):215-26. doi: 10.1016/s0168-1656(02)00133-5. J Biotechnol. 2002. PMID: 12141988 Review.
-
Drug:H+ antiporters in chemical stress response in yeast.Trends Microbiol. 2009 Jan;17(1):22-31. doi: 10.1016/j.tim.2008.09.007. Epub 2008 Dec 4. Trends Microbiol. 2009. PMID: 19062291 Review.
-
Yeast response and tolerance to benzoic acid involves the Gcn4- and Stp1-regulated multidrug/multixenobiotic resistance transporter Tpo1.Appl Microbiol Biotechnol. 2017 Jun;101(12):5005-5018. doi: 10.1007/s00253-017-8277-6. Epub 2017 Apr 13. Appl Microbiol Biotechnol. 2017. PMID: 28409382 Free PMC article.
Cited by
-
Some highlights of research on aging with invertebrates, 2010.Aging Cell. 2011 Feb;10(1):5-9. doi: 10.1111/j.1474-9726.2010.00649.x. Epub 2010 Dec 7. Aging Cell. 2011. PMID: 21078113 Free PMC article. Review.
-
Distinct segregation patterns of yeast cell-peripheral proteins uncovered by a method for protein segregatome analysis.Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):8909-8918. doi: 10.1073/pnas.1819715116. Epub 2019 Apr 11. Proc Natl Acad Sci U S A. 2019. PMID: 30975753 Free PMC article.
-
Sphingolipids facilitate age asymmetry of membrane proteins in dividing yeast cells.Mol Biol Cell. 2017 Oct 1;28(20):2712-2722. doi: 10.1091/mbc.E17-05-0335. Epub 2017 Aug 2. Mol Biol Cell. 2017. PMID: 28768828 Free PMC article.
-
Novel ABC Transporter Associated with Fluconazole Resistance in Aging of Cryptococcus neoformans.J Fungi (Basel). 2022 Jun 28;8(7):677. doi: 10.3390/jof8070677. J Fungi (Basel). 2022. PMID: 35887434 Free PMC article.
-
Mother-daughter asymmetry of pH underlies aging and rejuvenation in yeast.Elife. 2014 Sep 4;3:e03504. doi: 10.7554/eLife.03504. Elife. 2014. PMID: 25190112 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

