Transformation by a nucleotide-activated P2Y receptor is mediated by activation of Galphai, Galphaq and Rho-dependent signaling pathways
- PMID: 20653955
- PMCID: PMC2917412
- DOI: 10.1186/1750-2187-5-11
Transformation by a nucleotide-activated P2Y receptor is mediated by activation of Galphai, Galphaq and Rho-dependent signaling pathways
Abstract
Background: Nucleotide-actived P2Y receptors play critical roles in the growth of tumor cells by regulating cellular proliferation, differentiation and survival.
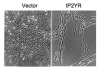
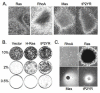
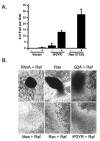
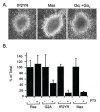
Results: Here we demonstrate that an avian P2Y purinoceptor (tP2YR) with unique pharmacological and signal transduction properties induces morphologic and growth transformation of rodent fibroblasts. tP2YR induced a transformed phenotype similar to the mas oncogene, a G protein-coupled receptor which causes transformation by activation of Rac-dependent pathways. tP2YR-transformed cells exhibited increased steady-state activation of Rac1 and RhoA. Like activated Rho GTPases, tP2YR cooperated with activated Raf and caused synergistic transformation of NIH3T3 cells. Our data indicate that the ability of tP2YR to cause transformation is due to its unique ability among purinergic receptors to simultaneously activate Galphaq and Galphai. Co-expression of constitutively activated mutants of these two Galpha subunits caused the same transformed phenotype as tP2YR and Mas. Furthermore, transformation by both tP2YR and Mas was blocked by pharmacological inhibition of GalphaI by pertussis toxin (PTX) indicating an essential role for Galphai in transformation by these G-protein coupled receptors.
Conclusions: Our data suggest that coordinated activation of Galphaq and Galphai may link the tP2YR and possibility the Mas oncogene with signaling pathways resulting in activation of Rho family proteins to promote cellular transformation.
Figures
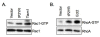
Similar articles
-
RhoA- and RhoD-dependent regulatory switch of Galpha subunit signaling by PAR-1 receptors in cellular invasion.FASEB J. 2002 Apr;16(6):565-76. doi: 10.1096/fj.01-0525com. FASEB J. 2002. PMID: 11919159
-
The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways.Oncogene. 2001 Apr 12;20(16):1953-63. doi: 10.1038/sj.onc.1204281. Oncogene. 2001. PMID: 11360179
-
On the selectivity of the Gαq inhibitor UBO-QIC: A comparison with the Gαi inhibitor pertussis toxin.Biochem Pharmacol. 2016 May 1;107:59-66. doi: 10.1016/j.bcp.2016.03.003. Epub 2016 Mar 5. Biochem Pharmacol. 2016. PMID: 26954502 Free PMC article.
-
G protein coupled receptor signaling through the Src and Stat3 pathway: role in proliferation and transformation.Oncogene. 2001 Mar 26;20(13):1601-6. doi: 10.1038/sj.onc.1204186. Oncogene. 2001. PMID: 11313907 Review.
-
Recommended Tool Compounds: Application of YM-254890 and FR900359 to Interrogate Gαq/11-Mediated Signaling Pathways.ACS Pharmacol Transl Sci. 2023 Nov 10;6(12):1790-1800. doi: 10.1021/acsptsci.3c00214. eCollection 2023 Dec 8. ACS Pharmacol Transl Sci. 2023. PMID: 38093837 Free PMC article. Review.
Cited by
-
Every day I'm rufflin': Calcium sensing and actin dynamics in the growth factor-independent membrane ruffling of professional phagocytes.Small GTPases. 2017 Apr 3;8(2):65-70. doi: 10.1080/21541248.2016.1197873. Epub 2016 Jun 7. Small GTPases. 2017. PMID: 27267709 Free PMC article. Review.
-
MAS C-Terminal Tail Interacting Proteins Identified by Mass Spectrometry- Based Proteomic Approach.PLoS One. 2015 Oct 20;10(10):e0140872. doi: 10.1371/journal.pone.0140872. eCollection 2015. PLoS One. 2015. PMID: 26484771 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected].Pharmacol Rev. 2015 Oct;67(4):754-819. doi: 10.1124/pr.114.010454. Pharmacol Rev. 2015. PMID: 26315714 Free PMC article. Review.
-
N-cadherin expression is regulated by UTP in schwannoma cells.Purinergic Signal. 2013 Jun;9(2):259-70. doi: 10.1007/s11302-012-9348-x. Epub 2012 Dec 28. Purinergic Signal. 2013. PMID: 23271561 Free PMC article.
-
Significance of angiotensin 1-7 coupling with MAS1 receptor and other GPCRs to the renin-angiotensin system: IUPHAR Review 22.Br J Pharmacol. 2017 May;174(9):737-753. doi: 10.1111/bph.13742. Epub 2017 Mar 9. Br J Pharmacol. 2017. PMID: 28194766 Free PMC article. Review.
References
-
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16:433–440. - PubMed
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

