Interferon regulatory factor-8-driven myeloid differentiation is regulated by 12/15-lipoxygenase-mediated redox signaling
- PMID: 20647030
- PMCID: PMC2963586
- DOI: 10.1016/j.exphem.2010.07.004
Interferon regulatory factor-8-driven myeloid differentiation is regulated by 12/15-lipoxygenase-mediated redox signaling
Abstract
Objective: Several transcription factors determine the cell fate decision between granulocytes and monocytes, but the upstream signal transduction pathways that govern myelopoiesis are largely unknown. Based on our observation of aberrant myeloid cell representation in hematopoietic tissues of 12/15-lipoxygenase (12/15-LOX)-deficient (Alox15) mice, we tested the hypothesis that polyunsaturated fatty acid metabolism regulates myelopoiesis.
Materials and methods: Multicolor flow cytometric analysis and methylcellulose assays were used to compare myelopoiesis and the differentiative capacity of progenitors from Alox15 and wild-type mice. Furthermore, we elucidated the mechanism by which 12/15-LOX is involved in regulation of myelopoiesis.
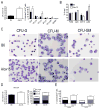
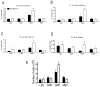
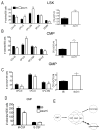
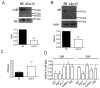
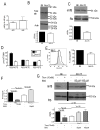
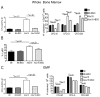
Results: Granulopoiesis in Alox15 mice is increased while monopoiesis is reduced. Moreover, there is an accumulation of granulocyte-macrophage progenitors that exhibit defective differentiation. Mechanistically, we demonstrate that transcriptional activity of interferon regulatory factor-8 (Irf8), which regulates myelopoiesis, is impaired in Alox15 progenitors and bone marrow-derived macrophages due to loss of 12/15-LOX-mediated redox regulation of Irf8 nuclear accumulation. Restoration of redox signaling in Alox15 bone marrow cells and granulocyte-macrophage progenitors reversed the defect in myeloid differentiation.
Conclusions: These data establish 12/15-LOX-mediated redox signaling as a novel regulator of myelopoiesis and Irf8.
Copyright © 2010 ISEH - Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
authors have no conflicting financial interests.
Figures
Similar articles
-
Lead Transiently Promotes Granulocyte-Macrophage Progenitor Differentiation and Subsequently Suppresses Common Myeloid Progenitor Differentiation.Toxicol Sci. 2017 Dec 1;160(2):268-283. doi: 10.1093/toxsci/kfx176. Toxicol Sci. 2017. PMID: 28973681
-
A reporter mouse reveals lineage-specific and heterogeneous expression of IRF8 during lymphoid and myeloid cell differentiation.J Immunol. 2014 Aug 15;193(4):1766-77. doi: 10.4049/jimmunol.1301939. Epub 2014 Jul 14. J Immunol. 2014. PMID: 25024380 Free PMC article.
-
IRF8 acts in lineage-committed rather than oligopotent progenitors to control neutrophil vs monocyte production.Blood. 2015 Feb 26;125(9):1452-9. doi: 10.1182/blood-2014-09-600833. Epub 2015 Jan 16. Blood. 2015. PMID: 25597637
-
Regulation of myelopoiesis by the transcription factor IRF8.Int J Hematol. 2015 Apr;101(4):342-51. doi: 10.1007/s12185-015-1761-9. Epub 2015 Mar 7. Int J Hematol. 2015. PMID: 25749660 Review.
-
Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases.Cell Mol Life Sci. 2018 Apr;75(8):1363-1376. doi: 10.1007/s00018-017-2724-5. Epub 2017 Dec 7. Cell Mol Life Sci. 2018. PMID: 29218601 Free PMC article. Review.
Cited by
-
12/15-Lipoxygenase Deficiency Impairs Neutrophil Granulopoiesis and Lung Proinflammatory Responses to Aspergillus fumigatus.J Immunol. 2020 Apr 1;204(7):1849-1858. doi: 10.4049/jimmunol.1900808. Epub 2020 Feb 26. J Immunol. 2020. PMID: 32102903 Free PMC article.
-
Myeloid 12/15-LOX regulates B cell numbers and innate immune antibody levels in vivo.Wellcome Open Res. 2017 Jan 4;2:1. doi: 10.12688/wellcomeopenres.10308.1. Wellcome Open Res. 2017. PMID: 28239665 Free PMC article.
-
Regulation of Tissue Inflammation by 12-Lipoxygenases.Biomolecules. 2021 May 11;11(5):717. doi: 10.3390/biom11050717. Biomolecules. 2021. PMID: 34064822 Free PMC article. Review.
-
The role of lipoxygenases in pathophysiology; new insights and future perspectives.Redox Biol. 2015 Dec;6:297-310. doi: 10.1016/j.redox.2015.08.006. Epub 2015 Aug 7. Redox Biol. 2015. PMID: 26298204 Free PMC article. Review.
-
Emerging Mechanisms and Disease Relevance of Ferroptosis.Trends Cell Biol. 2020 Jun;30(6):478-490. doi: 10.1016/j.tcb.2020.02.009. Epub 2020 Mar 21. Trends Cell Biol. 2020. PMID: 32413317 Free PMC article. Review.
References
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. - PubMed
-
- Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. - PubMed
-
- Koenigsmann J, Rudolph C, Sander S, Kershaw O, Gruber AD, Bullinger L, Schlegelberger B, Carstanjen D. Nf1 haploinsufficiency and Icsbp deficiency synergize in the development of leukemias. Blood. 2009;113:4690–4701. - PubMed
-
- Scheller M, Foerster J, Heyworth CM, Waring JF, Lohler J, Gilmore GL, Shadduck RK, Dexter TM, Horak I. Altered development and cytokine responses of myeloid progenitors in the absence of transcription factor, interferon consensus sequence binding protein. Blood. 1999;94:3764–3771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA091016-09/CA/NCI NIH HHS/United States
- P30CA10815/CA/NCI NIH HHS/United States
- T32CA09171/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- T32 GM007229/GM/NIGMS NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- T32GM07229/GM/NIGMS NIH HHS/United States
- R01CA091016/CA/NCI NIH HHS/United States
- T32 CA009171/CA/NCI NIH HHS/United States
- P30 ES013508-039001/ES/NIEHS NIH HHS/United States
- P30ES013508/ES/NIEHS NIH HHS/United States
- R01 CA091016/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

