Synthesis of aryl-heteroaryl ureas (AHUs) based on 4-aminoquinoline and their evaluation against the insulin-like growth factor receptor (IGF-1R)
- PMID: 20643554
- PMCID: PMC2918655
- DOI: 10.1016/j.bmc.2010.06.071
Synthesis of aryl-heteroaryl ureas (AHUs) based on 4-aminoquinoline and their evaluation against the insulin-like growth factor receptor (IGF-1R)
Abstract
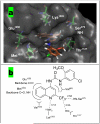
The insulin-like growth factor receptor (IGF-1R) is a receptor tyrosine kinase (RTK) involved in all stages of the development and propagation of breast and other cancers. The inhibition of IGF-1R by small molecules remains a promising strategy to treat cancer. Herein, we explore SAR around previously characterized lead compound (1), which is an aryl-heteroaryl urea (AHU) consisting of 4-aminoquinaldine and a substituted aromatic ring system. A library of novel AHU compounds was prepared based on derivatives of the 4-aminoquinoline heterocycle (including various 2-substituted derivatives, and naphthyridines). The compounds were screened for in vitro inhibitory activity against IGF-1R, and several compounds with improved activity (3-5 microM) were identified. Furthermore, a computational docking study was performed, which identifies a fairly consistent lowest energy mode of binding for the more-active set of inhibitors in this series, while the less-active inhibitors do not adopt a consistent mode of binding.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

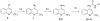

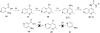
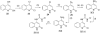
Similar articles
-
Parallel synthesis of diarylureas and their evaluation as inhibitors of insulin-like growth factor receptor.J Comb Chem. 2006 Sep-Oct;8(5):784-90. doi: 10.1021/cc050136z. J Comb Chem. 2006. PMID: 16961415
-
Synthesis and antiplasmodial activity of new heteroaryl derivatives of 7-chloro-4-aminoquinoline.Bioorg Med Chem. 2012 Oct 1;20(19):5965-79. doi: 10.1016/j.bmc.2012.07.040. Epub 2012 Aug 2. Bioorg Med Chem. 2012. PMID: 22917857
-
Kinase inhibitors of the IGF-1R as a potential therapeutic agent for rheumatoid arthritis.Autoimmunity. 2017 Aug;50(5):329-335. doi: 10.1080/08916934.2017.1344970. Epub 2017 Jul 6. Autoimmunity. 2017. PMID: 28682648
-
The rationale and development of therapeutic insulin-like growth factor axis inhibition for lung and other cancers.Clin Lung Cancer. 2009 Jul;10(4):262-72. doi: 10.3816/CLC.2009.n.037. Clin Lung Cancer. 2009. PMID: 19632946 Review.
-
Anti-insulin-like growth factor strategies in breast cancer.Semin Oncol. 2004 Feb;31(1 Suppl 3):54-63. doi: 10.1053/j.seminoncol.2004.01.007. Semin Oncol. 2004. PMID: 15052543 Review.
Cited by
-
Discovery of anthranilamides as a novel class of inhibitors of neurotropic alphavirus replication.Bioorg Med Chem. 2015 Apr 1;23(7):1569-87. doi: 10.1016/j.bmc.2015.01.054. Epub 2015 Feb 11. Bioorg Med Chem. 2015. PMID: 25740634 Free PMC article.
-
Optimized 4,5-Diarylimidazoles as Potent/Selective Inhibitors of Protein Kinase CK1δ and Their Structural Relation to p38α MAPK.Molecules. 2017 Mar 24;22(4):522. doi: 10.3390/molecules22040522. Molecules. 2017. PMID: 28338621 Free PMC article.
References
-
- Yuen JS, Macaulay VM. Expert Opin Ther Targets. 2008;12:589–603. - PubMed
-
- Bruchim I, Attias Z, Werner H. Expert Opin Ther Targets. 2009;13:1179–92. - PubMed
-
- Hofmann F, Garcia-Echeverria C. Drug Discov Today. 2005;10:1041–7. - PubMed
-
- Garcia-Echeverria C. IDrugs. 2006;9:415–9. - PubMed
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Cell. 1993;75:73–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

