Cytoplasmic viral replication complexes
- PMID: 20638644
- PMCID: PMC2921950
- DOI: 10.1016/j.chom.2010.06.010
Cytoplasmic viral replication complexes
Abstract
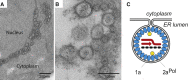
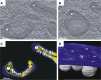
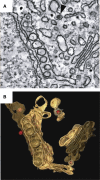
Many viruses that replicate in the cytoplasm compartmentalize their genome replication and transcription in organelle-like structures that enhance replication efficiency and protection from host defenses. In particular, recent studies with diverse positive-strand RNA viruses have further elucidated the ultrastructure of membrane-bound RNA replication complexes and how these complexes function in close coordination with virion assembly and budding. The structure, function, and assembly of some positive-strand RNA virus replication complexes have parallels and potential evolutionary links with the replicative cores of double-strand RNA virus and retrovirus virions and more general similarities with the replication factories of cytoplasmic DNA viruses.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
DNA virus replication compartments.J Virol. 2014 Feb;88(3):1404-20. doi: 10.1128/JVI.02046-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257611 Free PMC article. Review.
-
Virus evolution: fitting lifestyles to a T.Curr Biol. 2005 Jun 21;15(12):R465-7. doi: 10.1016/j.cub.2005.06.016. Curr Biol. 2005. PMID: 15964268 Review.
-
Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication.Proc Natl Acad Sci U S A. 2004 Aug 3;101(31):11263-8. doi: 10.1073/pnas.0404157101. Epub 2004 Jul 27. Proc Natl Acad Sci U S A. 2004. PMID: 15280537 Free PMC article.
-
Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories.Annu Rev Microbiol. 2010;64:241-56. doi: 10.1146/annurev.micro.112408.134012. Annu Rev Microbiol. 2010. PMID: 20825348 Review.
-
Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses.Nat Rev Microbiol. 2006 May;4(5):371-82. doi: 10.1038/nrmicro1389. Nat Rev Microbiol. 2006. PMID: 16582931 Free PMC article. Review.
Cited by
-
Five questions on the cell-to-cell movement of Orthotospoviruses.BBA Adv. 2024 Oct 16;6:100124. doi: 10.1016/j.bbadva.2024.100124. eCollection 2024. BBA Adv. 2024. PMID: 39498475 Free PMC article.
-
The roles and mechanisms of endoplasmic reticulum stress-mediated autophagy in animal viral infections.Vet Res. 2024 Sep 3;55(1):107. doi: 10.1186/s13567-024-01360-4. Vet Res. 2024. PMID: 39227990 Free PMC article. Review.
-
Mouse guanylate-binding proteins of the chromosome 3 cluster do not mediate antiviral activity in vitro or in mouse models of infection.Commun Biol. 2024 Aug 25;7(1):1050. doi: 10.1038/s42003-024-06748-8. Commun Biol. 2024. PMID: 39183326 Free PMC article.
-
The astrovirus N-terminal nonstructural protein anchors replication complexes to the perinuclear ER membranes.PLoS Pathog. 2024 Jul 15;20(7):e1011959. doi: 10.1371/journal.ppat.1011959. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39008516 Free PMC article.
-
Megataxonomy and global ecology of the virosphere.ISME J. 2024 Jan 8;18(1):wrad042. doi: 10.1093/ismejo/wrad042. ISME J. 2024. PMID: 38365236 Free PMC article. Review.
References
-
- Annamalai P., Rao A.L. Replication-independent expression of genome components and capsid protein of brome mosaic virus in planta: a functional role for viral replicase in RNA packaging. Virology. 2005;338:96–111. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

