Mechanisms that determine plasma cell lifespan and the duration of humoral immunity
- PMID: 20636813
- PMCID: PMC7165522
- DOI: 10.1111/j.1600-065X.2010.00912.x
Mechanisms that determine plasma cell lifespan and the duration of humoral immunity
Erratum in
- Immunol Rev. 2010 Sep;237(1):284
Abstract
Humoral immunity following vaccination or infection is mainly derived from two types of cells: memory B cells and plasma cells. Memory B cells do not actively secrete antibody but instead maintain their immunoglobulin in the membrane-bound form that serves as the antigen-specific B-cell receptor. In contrast, plasma cells are terminally differentiated cells that no longer express surface-bound immunoglobulin but continuously secrete antibody without requiring further antigenic stimulation. Pre-existing serum or mucosal antibody elicited by plasma cells (or other intermediate antibody-secreting cells) represents the first line of defense against reinfection and is critical for protection against many microbial diseases. However, the mechanisms involved with maintaining long-term antibody production are not fully understood. Here, we examine several models of long-term humoral immunity and present a new model, described as the 'Imprinted Lifespan' model of plasma cell longevity. The foundation of this model is that plasma cells are imprinted with a predetermined lifespan based on the magnitude of B-cell signaling that occurs during the induction of an antigen-specific humoral immune response. This represents a testable hypothesis and may explain why some antigen-specific antibody responses fade over time whereas others are maintained essentially for life.
Figures
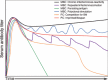
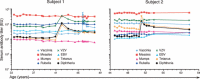


Similar articles
-
Functional Enrichment and Analysis of Antigen-Specific Memory B Cell Antibody Repertoires in PBMCs.Front Immunol. 2019 Jun 25;10:1452. doi: 10.3389/fimmu.2019.01452. eCollection 2019. Front Immunol. 2019. PMID: 31293598 Free PMC article.
-
Selective depletion of plasma cells in vivo based on the specificity of their secreted antibodies.Eur J Immunol. 2020 Feb;50(2):284-291. doi: 10.1002/eji.201948144. Epub 2019 Nov 12. Eur J Immunol. 2020. PMID: 31714996
-
[Plasma cells].Z Rheumatol. 2015 Feb;74(1):20-5. doi: 10.1007/s00393-014-1438-4. Z Rheumatol. 2015. PMID: 25616773 German.
-
Non-classical B Cell Memory of Allergic IgE Responses.Front Immunol. 2019 Apr 26;10:715. doi: 10.3389/fimmu.2019.00715. eCollection 2019. Front Immunol. 2019. PMID: 31105687 Free PMC article. Review.
-
Maintenance of serum antibody levels.Annu Rev Immunol. 2005;23:367-86. doi: 10.1146/annurev.immunol.23.021704.115723. Annu Rev Immunol. 2005. PMID: 15771575 Review.
Cited by
-
Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset.Blood. 2020 Nov 26;136(22):2588-2591. doi: 10.1182/blood.2020008367. Blood. 2020. PMID: 33001206 Free PMC article. Clinical Trial.
-
[Plasma cell-eosinophil interaction].Z Rheumatol. 2013 Apr;72(3):267-9. doi: 10.1007/s00393-012-1032-6. Z Rheumatol. 2013. PMID: 23503783 German. No abstract available.
-
Antigen-specific B memory cell responses to Plasmodium falciparum malaria antigens and Schistosoma haematobium antigens in co-infected Malian children.PLoS One. 2012;7(6):e37868. doi: 10.1371/journal.pone.0037868. Epub 2012 Jun 5. PLoS One. 2012. PMID: 22693628 Free PMC article.
-
A proof-of-concept study for the design of a VLP-based combinatorial HPV and placental malaria vaccine.Sci Rep. 2019 Mar 27;9(1):5260. doi: 10.1038/s41598-019-41522-5. Sci Rep. 2019. PMID: 30918267 Free PMC article.
-
Preformed frequencies of cytomegalovirus (CMV)-specific memory T and B cells identify protected CMV-sensitized individuals among seronegative kidney transplant recipients.Clin Infect Dis. 2014 Dec 1;59(11):1537-45. doi: 10.1093/cid/ciu589. Epub 2014 Jul 21. Clin Infect Dis. 2014. PMID: 25048845 Free PMC article.
References
-
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007;357:1903–1915. - PubMed
-
- Jerne NK. Idiotypic networks and other preconceived ideas. Immunol Rev 1984;79:5–24. - PubMed
-
- MacLennan IC, Casamayor‐Palleja M, Toellner KM, Gulbranson‐Judge A, Gordon J. Memory B‐cell clones and the diversity of their members. Semin Immunol 1997;9:229–234. - PubMed
-
- Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989;159:1000–1001. - PubMed
-
- Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med 1991;325:1545–1550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

