Programmed cell death of dendritic cells in immune regulation
- PMID: 20636805
- PMCID: PMC3282617
- DOI: 10.1111/j.1600-065X.2010.00916.x
Programmed cell death of dendritic cells in immune regulation
Abstract
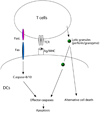
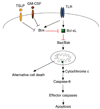
Programmed cell death is essential for the maintenance of lymphocyte homeostasis and immune tolerance. Dendritic cells (DCs), the most efficient antigen-presenting cells, represent a small cell population in the immune system. However, DCs play major roles in the regulation of both innate and adaptive immune responses. Programmed cell death in DCs is essential for regulating DC homeostasis and consequently, the scope of immune responses. Interestingly, different DC subsets show varied turnover rates in vivo. The conventional DCs are relatively short-lived in most lymphoid organs, while plasmacytoid DCs are long-lived cells. Mitochondrion-dependent programmed cell death plays an important role in regulating spontaneous DC turnover. Antigen-specific T cells are also capable of killing DCs, thereby providing a mechanism for negative feedback regulation of immune responses. It has been shown that a surplus of DCs due to defects in programmed cell death leads to overactivation of lymphocytes and the onset of autoimmunity. Studying programmed cell death in DCs will shed light on the roles for DC turnover in the regulation of the duration and magnitude of immune responses in vivo and in the maintenance of immune tolerance.
Figures

Similar articles
-
Regulation of Immune Responses by Spontaneous and T cell-mediated Dendritic Cell Death.J Clin Cell Immunol. 2011 Dec 11;S3:005. doi: 10.4172/2155-9899.S3-005. J Clin Cell Immunol. 2011. PMID: 22468233 Free PMC article.
-
Dendritic type, accessory cells within the mammalian thymic microenvironment. Antigen presentation in the dendritic neuro-endocrine-immune cellular network.In Vivo. 1997 Jul-Aug;11(4):351-70. In Vivo. 1997. PMID: 9292303
-
Immune regulation through mitochondrion-dependent dendritic cell death induced by T regulatory cells.J Immunol. 2011 Dec 1;187(11):5684-92. doi: 10.4049/jimmunol.1101834. Epub 2011 Oct 26. J Immunol. 2011. PMID: 22031758 Free PMC article.
-
Dendritic cell apoptosis: regulation of tolerance versus immunity.J Immunol. 2010 Jul 15;185(2):795-802. doi: 10.4049/jimmunol.1000325. J Immunol. 2010. PMID: 20601611 Review.
-
Dendritic cell subsets in T cell programming: location dictates function.Nat Rev Immunol. 2019 Feb;19(2):89-103. doi: 10.1038/s41577-018-0088-1. Nat Rev Immunol. 2019. PMID: 30464294 Free PMC article. Review.
Cited by
-
Impaired functions of human monocyte-derived dendritic cells and induction of regulatory T cells by pathogenic Leptospira.PLoS Negl Trop Dis. 2023 Nov 20;17(11):e0011781. doi: 10.1371/journal.pntd.0011781. eCollection 2023 Nov. PLoS Negl Trop Dis. 2023. PMID: 37983293 Free PMC article.
-
Tsc1 expression by dendritic cells is required to preserve T-cell homeostasis and response.Cell Death Dis. 2017 Jan 12;8(1):e2553. doi: 10.1038/cddis.2016.487. Cell Death Dis. 2017. PMID: 28079897 Free PMC article.
-
Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response.J Transl Med. 2017 Jan 3;15(1):1. doi: 10.1186/s12967-016-1111-6. J Transl Med. 2017. PMID: 28049494 Free PMC article.
-
Dendritic cells resist to disulfiram-induced cytotoxicity, but reduced interleukin-12/23(p40) production.Korean J Physiol Pharmacol. 2023 Sep 1;27(5):471-479. doi: 10.4196/kjpp.2023.27.5.471. Korean J Physiol Pharmacol. 2023. PMID: 37641809 Free PMC article.
-
Effects of Hypericum Perforatum, in a rodent model of periodontitis.BMC Complement Altern Med. 2010 Nov 23;10:73. doi: 10.1186/1472-6882-10-73. BMC Complement Altern Med. 2010. PMID: 21092263 Free PMC article.
References
-
- Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. - PubMed
-
- Gaudin E, Rosado M, Agenes F, McLean A, Freitas AA. B-cell homeostasis, competition, resources, and positive selection by self-antigens. Immunol Rev. 2004;197:102–115. - PubMed
-
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

