Fosfomycin suppresses RS-virus-induced Streptococcus pneumoniae and Haemophilus influenzae adhesion to respiratory epithelial cells via the platelet-activating factor receptor
- PMID: 20629755
- PMCID: PMC7110074
- DOI: 10.1111/j.1574-6968.2010.02049.x
Fosfomycin suppresses RS-virus-induced Streptococcus pneumoniae and Haemophilus influenzae adhesion to respiratory epithelial cells via the platelet-activating factor receptor
Abstract
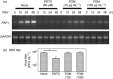
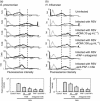
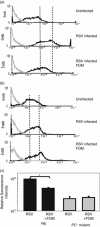
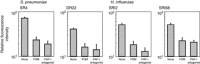
Human respiratory syncytial virus (RSV) sometimes causes acute and severe lower respiratory tract illness in infants and young children. The platelet-activating factor (PAF) receptor, which is a receptor for Streptococcus pneumoniae and Haemophilus influenzae, is upregulated by RSV infection in the pulmonary epithelial cell line A549. Fosfomycin, an antimicrobial agent, significantly suppressed PAF receptor induction by RSV infection at the mRNA and cell surface expression levels. Fosfomycin also suppressed RSV-induced adhesion of fluorescence-labeled S. pneumoniae and H. influenzae cells, as determined by flow cytometry and fluorescence microscopy. The RSV-induced bacterial adhesion was suggested to be host-PAF-receptor and bacterial-phosphocholine mediated. Fosfomycin, which has been shown to exhibit antimicrobial and immunomodulatory activities, was found here to suppress adhesion by disease-causing bacteria. Thus, fosfomycin might prevent secondary bacterial infection during RSV infection.
Figures


Similar articles
-
Clarithromycin suppresses human respiratory syncytial virus infection-induced Streptococcus pneumoniae adhesion and cytokine production in a pulmonary epithelial cell line.Mediators Inflamm. 2012;2012:528568. doi: 10.1155/2012/528568. Epub 2012 Jun 12. Mediators Inflamm. 2012. PMID: 22761540 Free PMC article.
-
An antagonist of the platelet-activating factor receptor inhibits adherence of both nontypeable Haemophilus influenzae and Streptococcus pneumoniae to cultured human bronchial epithelial cells exposed to cigarette smoke.Int J Chron Obstruct Pulmon Dis. 2016 Jul 25;11:1647-55. doi: 10.2147/COPD.S108698. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27524890 Free PMC article.
-
Role of phosphorylcholine in Streptococcus pneumoniae and nontypeable Haemophilus influenzae adherence to epithelial cells.Auris Nasus Larynx. 2019 Aug;46(4):513-519. doi: 10.1016/j.anl.2018.11.003. Epub 2018 Nov 29. Auris Nasus Larynx. 2019. PMID: 30503566
-
Does upregulated host cell receptor expression provide a link between bacterial adhesion and chronic respiratory disease?J Transl Med. 2016 Oct 26;14(1):304. doi: 10.1186/s12967-016-1063-x. J Transl Med. 2016. PMID: 27782846 Free PMC article. Review.
-
The platelet activating factor receptor: a new anti-infective target in respiratory disease?Thorax. 2012 Sep;67(9):840-1. doi: 10.1136/thoraxjnl-2012-202206. Epub 2012 Jul 16. Thorax. 2012. PMID: 22802333 Review.
Cited by
-
Clarithromycin suppresses human respiratory syncytial virus infection-induced Streptococcus pneumoniae adhesion and cytokine production in a pulmonary epithelial cell line.Mediators Inflamm. 2012;2012:528568. doi: 10.1155/2012/528568. Epub 2012 Jun 12. Mediators Inflamm. 2012. PMID: 22761540 Free PMC article.
-
Fosfomycin.Clin Microbiol Rev. 2016 Apr;29(2):321-47. doi: 10.1128/CMR.00068-15. Clin Microbiol Rev. 2016. PMID: 26960938 Free PMC article. Review.
-
Antibiotic Resistance to Molecules Commonly Prescribed for the Treatment of Antibiotic-Resistant Gram-Positive Pathogens: What Is Relevant for the Clinician?Pathogens. 2024 Jan 19;13(1):88. doi: 10.3390/pathogens13010088. Pathogens. 2024. PMID: 38276161 Free PMC article. Review.
-
The Respiratory Microbiome in Paediatric Chronic Wet Cough: What Is Known and Future Directions.J Clin Med. 2023 Dec 28;13(1):171. doi: 10.3390/jcm13010171. J Clin Med. 2023. PMID: 38202177 Free PMC article. Review.
-
Impact of the glpQ2 gene on virulence in a Streptococcus pneumoniae serotype 19A sequence type 320 strain.Infect Immun. 2015 Feb;83(2):682-92. doi: 10.1128/IAI.02357-14. Epub 2014 Nov 24. Infect Immun. 2015. PMID: 25422269 Free PMC article.
References
-
- Andrade MA, Hoberman A, Glustein J, Paradise JL, Wald ER. (1998) Acute otitis media in children with bronchiolitis. Pediatrics 101: 617–619. - PubMed
-
- Assmann G, Schriewer H. (1985) Choline. Methods in Enzymatic Analysis, Vol. VIII (Bergmeyer H, Bergmeyer J, Grassl M, eds), pp. 106–111. Verlag Chemie, Weinheim.
-
- Chonmaitree T, Heikkinen T. (2000) Viruses and acute otitis media. Pediatr Infect Dis J 19: 1005–1007. - PubMed
-
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. (1995) Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377: 435–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

