Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01
- PMID: 20616231
- PMCID: PMC2981354
- DOI: 10.1126/science.1192819
Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01
Abstract
During HIV-1 infection, antibodies are generated against the region of the viral gp120 envelope glycoprotein that binds CD4, the primary receptor for HIV-1. Among these antibodies, VRC01 achieves broad neutralization of diverse viral strains. We determined the crystal structure of VRC01 in complex with a human immunodeficiency virus HIV-1 gp120 core. VRC01 partially mimics CD4 interaction with gp120. A shift from the CD4-defined orientation, however, focuses VRC01 onto the vulnerable site of initial CD4 attachment, allowing it to overcome the glycan and conformational masking that diminishes the neutralization potency of most CD4-binding-site antibodies. To achieve this recognition, VRC01 contacts gp120 mainly through immunoglobulin V-gene regions substantially altered from their genomic precursors. Partial receptor mimicry and extensive affinity maturation thus facilitate neutralization of HIV-1 by natural human antibodies.
Figures




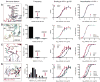
 ” marks a site of N-linked glycosylation, “
” marks a site of N-linked glycosylation, “ ” for cysteine residues involved in a non-canonical disulfide, and “
” for cysteine residues involved in a non-canonical disulfide, and “ ” if the residue has been deleted during affinity maturation. In B-E, unusual features of VRC01 are shown structurally (far left panel), in terms of frequency as a histogram with other antibodies (second panel from left), and in the context of affinity meaurements after mutational alteration (right two panels). Affinity measurements were made by ELISA to the gp120 construct used in crystallization (93TH057) and to a disulfide stabilized HXBc2 core (22). (B) N-linked glycosylation. The conserved tri-mannose core is shown with observed electron density, along with frequency and effect of removal on affinity. (C) Extra disulfide. Variable heavy domains naturally have two Cys, linked by a disulfide; VRC01 has an extra disulfide linking CDR H1 and H3 regions. This occurs rarely in antibodies, but its removal by mutation to Ser/Ala has little effect on affinity. (D) CDR L1 deletion. A two amino acid deletion in the CDR L1, prevents potential clashes with loop D of gp120. Such deletions are rarely observed; reversion to the longer loop may have a 10-100-fold effect on gp120 affinity. (E) Somatically altered contact surface. The far left panel shows the VRC01 light chain in violet and heavy chain in green. Residues altered by affinity maturation are depicted with “balls” and contacts with HIV-1 gp120 are colored red. About half the contacts are altered during the maturation process. Analysis of human antibody-protein complexes in the protein-data bank shows this degree of contact surface alteration is rare; reversion of each of the contact site to genome has little effect (Table S12), though in aggregate the effect on affinity is larger.
” if the residue has been deleted during affinity maturation. In B-E, unusual features of VRC01 are shown structurally (far left panel), in terms of frequency as a histogram with other antibodies (second panel from left), and in the context of affinity meaurements after mutational alteration (right two panels). Affinity measurements were made by ELISA to the gp120 construct used in crystallization (93TH057) and to a disulfide stabilized HXBc2 core (22). (B) N-linked glycosylation. The conserved tri-mannose core is shown with observed electron density, along with frequency and effect of removal on affinity. (C) Extra disulfide. Variable heavy domains naturally have two Cys, linked by a disulfide; VRC01 has an extra disulfide linking CDR H1 and H3 regions. This occurs rarely in antibodies, but its removal by mutation to Ser/Ala has little effect on affinity. (D) CDR L1 deletion. A two amino acid deletion in the CDR L1, prevents potential clashes with loop D of gp120. Such deletions are rarely observed; reversion to the longer loop may have a 10-100-fold effect on gp120 affinity. (E) Somatically altered contact surface. The far left panel shows the VRC01 light chain in violet and heavy chain in green. Residues altered by affinity maturation are depicted with “balls” and contacts with HIV-1 gp120 are colored red. About half the contacts are altered during the maturation process. Analysis of human antibody-protein complexes in the protein-data bank shows this degree of contact surface alteration is rare; reversion of each of the contact site to genome has little effect (Table S12), though in aggregate the effect on affinity is larger.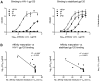
Comment in
-
AIDS/HIV. A boost for HIV vaccine design.Science. 2010 Aug 13;329(5993):770-3. doi: 10.1126/science.1194693. Science. 2010. PMID: 20705840 No abstract available.
Similar articles
-
Increasing the potency and breadth of an HIV antibody by using structure-based rational design.Science. 2011 Dec 2;334(6060):1289-93. doi: 10.1126/science.1213782. Epub 2011 Oct 27. Science. 2011. PMID: 22033520 Free PMC article.
-
Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing.Science. 2011 Sep 16;333(6049):1593-602. doi: 10.1126/science.1207532. Epub 2011 Aug 11. Science. 2011. PMID: 21835983 Free PMC article.
-
Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding.Science. 2011 Sep 16;333(6049):1633-7. doi: 10.1126/science.1207227. Epub 2011 Jul 14. Science. 2011. PMID: 21764753 Free PMC article.
-
Structural Features of Broadly Neutralizing Antibodies and Rational Design of Vaccine.Adv Exp Med Biol. 2018;1075:73-95. doi: 10.1007/978-981-13-0484-2_4. Adv Exp Med Biol. 2018. PMID: 30030790 Review.
-
Biological consequences of human immunodeficiency virus type 1 envelope polymorphism: does variation matter? 1995 Fleming Lecture.J Gen Virol. 1996 Dec;77 ( Pt 12):2905-19. doi: 10.1099/0022-1317-77-12-2905. J Gen Virol. 1996. PMID: 9000081 Review. No abstract available.
Cited by
-
Identification of Non-HIV Immunogens That Bind to Germline b12 Predecessors and Prime for Elicitation of Cross-clade Neutralizing HIV-1 Antibodies.PLoS One. 2015 May 26;10(5):e0126428. doi: 10.1371/journal.pone.0126428. eCollection 2015. PLoS One. 2015. PMID: 26010511 Free PMC article.
-
Structural plasticity and conformational transitions of HIV envelope glycoprotein gp120.PLoS One. 2012;7(12):e52170. doi: 10.1371/journal.pone.0052170. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300605 Free PMC article.
-
HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies.J Virol. 2015 Apr;89(8):4201-13. doi: 10.1128/JVI.03608-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631091 Free PMC article.
-
VRC01 antibody protects against vaginal and rectal transmission of human immunodeficiency virus 1 in hu-BLT mice.Arch Virol. 2016 Sep;161(9):2449-55. doi: 10.1007/s00705-016-2942-4. Epub 2016 Jun 24. Arch Virol. 2016. PMID: 27343044 Free PMC article.
-
Rational HIV immunogen design to target specific germline B cell receptors.Science. 2013 May 10;340(6133):711-6. doi: 10.1126/science.1234150. Epub 2013 Mar 28. Science. 2013. PMID: 23539181 Free PMC article.
References
-
- Weiss RA, et al. Nature. 1985 Jul 4-10;316:69. - PubMed
-
- Mascola JR, Montefiori DC. Annu Rev Immunol. 2010 Mar;28:413. - PubMed
-
- Burton DR. Nat Rev Immunol. 2002 Sep;2:706. - PubMed
-
- Burton DR, et al. Nat Immunol. 2004 Mar;5:233. - PubMed
-
-
Antibodies 2F5 and 4E10 require non-specific membrane interaction (34-35), antibody 2G12 recognizes carbohydrate and is domain swapped (32-33), and antibody b12 was originally derived by phage display and has heavy-chain-only recognition (22).
-
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

