Differential control of TAp73 and DeltaNp73 protein stability by the ring finger ubiquitin ligase PIR2
- PMID: 20615966
- PMCID: PMC2919933
- DOI: 10.1073/pnas.0911828107
Differential control of TAp73 and DeltaNp73 protein stability by the ring finger ubiquitin ligase PIR2
Abstract
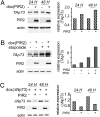
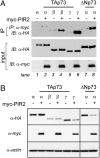
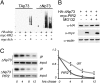
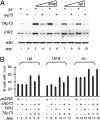
p73 is a p53-related transcription factor with fundamental roles in development and tumor suppression. Transcription from two different promoters on the p73 gene results in generation of transcriptionally active TAp73 isoforms and dominant negative DeltaNp73 isoforms with opposing pro- and anti-apoptotic functions. Therefore, the relative ratio of each isoform is an important determinant of the cell fate. Proteasomal degradation of p73 is mediated by polyubiquitination-dependent and -independent processes both of which appear, thus far, to lack selectivity for the TAp73 and DeltaNp73 isoforms. Here, we describe the characterization of another transcriptional target of TAp73; a ring finger domain ubiquitin ligase p73 Induced RING 2 protein (PIR2). Although PIR2 was initially identified a p53-induced gene (p53RFP), low abundance of PIR2 transcript in mouse embryonic fibroblasts of TAp73 KO mice compared with WT mice and comparison of PIR2 mRNA and protein levels following TAp73 or p53 overexpression substantiate TAp73 isoforms as strong inducers of PIR2. Although PIR2 expression was induced by DNA damage, its expression did not alter apoptotic response or cell cycle profile per se. However, coexpression of PIR2 with TAp73 or DeltaNp73 resulted in an increase of the TA/DeltaNp73 ratio, due to preferential degradation of DeltaNp73. Finally, PIR2 was able to relieve the inhibitory effect of DeltaNp73 on TAp73 induced apoptosis following DNA damage. These results suggest that PIR2, by being induced by TAp73 and degrading DeltaNp73, differentially regulates TAp73/DeltaNp73 stability, and, hence, it may offer a therapeutic approach to enhance the chemosensitivity of tumor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Apoptin induces apoptosis by changing the equilibrium between the stability of TAp73 and ΔNp73 isoforms through ubiquitin ligase PIR2.Apoptosis. 2012 Aug;17(8):762-76. doi: 10.1007/s10495-012-0720-7. Apoptosis. 2012. PMID: 22484480
-
The p73 tumor suppressor is targeted by Pirh2 RING finger E3 ubiquitin ligase for the proteasome-dependent degradation.J Biol Chem. 2011 Oct 14;286(41):35388-35395. doi: 10.1074/jbc.M111.261537. Epub 2011 Aug 18. J Biol Chem. 2011. PMID: 21852228 Free PMC article.
-
TAp73 and ΔNp73 have opposing roles in 5-aza-2'-deoxycytidine-induced apoptosis in breast cancer cells.Mol Cells. 2014 Aug;37(8):605-12. doi: 10.14348/molcells.2014.0154. Epub 2014 Aug 18. Mol Cells. 2014. PMID: 25134538 Free PMC article.
-
p73 induces apoptosis by different mechanisms.Biochem Biophys Res Commun. 2005 Jun 10;331(3):713-7. doi: 10.1016/j.bbrc.2005.03.156. Biochem Biophys Res Commun. 2005. PMID: 15865927 Review.
-
Regulation of the p73 protein stability and degradation.Biochem Biophys Res Commun. 2005 Jun 10;331(3):707-12. doi: 10.1016/j.bbrc.2005.03.158. Biochem Biophys Res Commun. 2005. PMID: 15865926 Review.
Cited by
-
Analysis of the oligomeric state and transactivation potential of TAp73α.Cell Death Differ. 2013 Aug;20(8):1008-16. doi: 10.1038/cdd.2013.23. Epub 2013 Mar 29. Cell Death Differ. 2013. PMID: 23538419 Free PMC article.
-
The E2F4/p130 Repressor Complex Cooperates with Oncogenic ΔNp73α To Inhibit Gene Expression in Human Papillomavirus 38 E6/E7-Transformed Keratinocytes and in Cancer Cells.mSphere. 2023 Apr 20;8(2):e0005623. doi: 10.1128/msphere.00056-23. Epub 2023 Mar 8. mSphere. 2023. PMID: 36883841 Free PMC article.
-
High ΔNp73/TAp73 ratio is associated with poor prognosis in acute promyelocytic leukemia.Blood. 2015 Nov 12;126(20):2302-6. doi: 10.1182/blood-2015-01-623330. Epub 2015 Oct 1. Blood. 2015. PMID: 26429976 Free PMC article. Clinical Trial.
-
Mechanisms of Functional Pleiotropy of p73 in Cancer and Beyond.Front Cell Dev Biol. 2021 Sep 28;9:737735. doi: 10.3389/fcell.2021.737735. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34650986 Free PMC article. Review.
-
Structure and apoptotic function of p73.BMB Rep. 2015 Feb;48(2):81-90. doi: 10.5483/bmbrep.2015.48.2.255. BMB Rep. 2015. PMID: 25441426 Free PMC article. Review.
References
-
- Melino G, Lu X, Gasco M, Crook T, Knight RA. Functional regulation of p73 and p63: Development and cancer. Trends Biochem Sci. 2003;28:663–670. - PubMed
-
- Melino G, De Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2:605–615. - PubMed
-
- Ishimoto O, et al. Possible oncogenic potential of DeltaNp73: A newly identified isoform of human p73. Cancer Res. 2002;62:636–641. - PubMed
-
- Stiewe T, Theseling CC, Pützer BM. Transactivation-deficient Delta TA-p73 inhibits p53 by direct competition for DNA binding: Implications for tumorigenesis. J Biol Chem. 2002;277:14177–14185. - PubMed
-
- Pozniak CD, et al. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science. 2000;289:304–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

