Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models
- PMID: 20615072
- PMCID: PMC3025764
- DOI: 10.1089/ars.2010.3370
Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models
Abstract
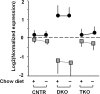
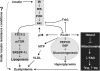
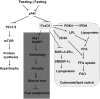
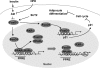
Forkhead box O (FOXO) transcription factors have been implicated in regulating the metabolism, cellular proliferation, stress resistance, apoptosis, and longevity. Through the insulin receptor substrate → phosphoinositide 3-kinase → Akt signal cascade, FOXO integrates insulin action with the systemic nutrient and energy homeostasis. Activation of FOXO1 in liver induces gluconeogenesis via phosphoenolpyruvate carboxykinase (PEPCK)/glucose 6-phosphate pathway, and disrupts mitochondrial metabolism and lipid metabolism via heme oxygenase 1/sirtuin 1/Ppargc1α pathway. In skeletal muscle, FOXO1 activation underpins the carbohydrate/lipid switch during fasting state. Inhibition of FOXO1 under physiological conditions accounts for maintenance of skeletal muscle mass/function and adipose differentiation. In pancreatic β-cells, nuclear translocation of FOXO1 antagonizes pancreatic and duodenal homeobox 1 and attenuates β-cells proliferation and insulin secretion. Regardless, FOXO1 promotes the proliferation of β-cells through induction of Cyclin D1 in low nutrition, and elicits antioxidant mechanism to protect against β-cell failure during oxidative insults. In the brain, FOXO1 controls food intake through transcriptional regulation of the orexigenic neuropeptide Y, agouti-related protein, and carboxypeptidase E. In this article, we review the role of FOXO1 in the regulation of metabolism and energy expenditure based on recent findings from mouse models, and discuss the therapeutic value of targeting FOXO1 in metabolic diseases.
Figures

Similar articles
-
Overexpression of FoxO1 in the hypothalamus and pancreas causes obesity and glucose intolerance.Endocrinology. 2012 Feb;153(2):659-71. doi: 10.1210/en.2011-1635. Epub 2011 Dec 20. Endocrinology. 2012. PMID: 22186407
-
FoxO1, the transcriptional chief of staff of energy metabolism.Bone. 2012 Feb;50(2):437-43. doi: 10.1016/j.bone.2011.06.034. Epub 2011 Jul 28. Bone. 2012. PMID: 21816244 Free PMC article. Review.
-
Forkhead box O1/pancreatic and duodenal homeobox 1 intracellular translocation is regulated by c-Jun N-terminal kinase and involved in prostaglandin E2-induced pancreatic beta-cell dysfunction.Endocrinology. 2009 Dec;150(12):5284-93. doi: 10.1210/en.2009-0671. Epub 2009 Oct 16. Endocrinology. 2009. PMID: 19837872
-
Acute exercise reduces hepatic glucose production through inhibition of the Foxo1/HNF-4alpha pathway in insulin resistant mice.J Physiol. 2010 Jun 15;588(Pt 12):2239-53. doi: 10.1113/jphysiol.2009.183996. Epub 2010 Apr 26. J Physiol. 2010. PMID: 20421289 Free PMC article.
-
The role of FOXO in the regulation of metabolism.Curr Diab Rep. 2009 Jun;9(3):208-14. doi: 10.1007/s11892-009-0034-5. Curr Diab Rep. 2009. PMID: 19490822 Review.
Cited by
-
Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne.Exp Dermatol. 2013 May;22(5):311-5. doi: 10.1111/exd.12142. Exp Dermatol. 2013. PMID: 23614736 Free PMC article.
-
Moderate alcohol consumption diminishes the development of non-alcoholic fatty liver disease (NAFLD) in ob/ob mice.Eur J Nutr. 2016 Apr;55(3):1153-64. doi: 10.1007/s00394-015-0929-7. Epub 2015 May 24. Eur J Nutr. 2016. PMID: 26003186
-
Benzylglucosinolate Derived Isothiocyanate from Tropaeolum majus Reduces Gluconeogenic Gene and Protein Expression in Human Cells.PLoS One. 2016 Sep 13;11(9):e0162397. doi: 10.1371/journal.pone.0162397. eCollection 2016. PLoS One. 2016. PMID: 27622707 Free PMC article.
-
SIRT1: new avenues of discovery for disorders of oxidative stress.Expert Opin Ther Targets. 2012 Feb;16(2):167-78. doi: 10.1517/14728222.2012.648926. Epub 2012 Jan 10. Expert Opin Ther Targets. 2012. PMID: 22233091 Free PMC article. Review.
-
Phytochemicals targeting NAFLD through modulating the dual function of forkhead box O1 (FOXO1) transcription factor signaling pathways.Naunyn Schmiedebergs Arch Pharmacol. 2022 Jul;395(7):741-755. doi: 10.1007/s00210-022-02234-2. Epub 2022 Mar 31. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35357518 Review.
References
-
- Ai J. Duan J. Lv X. Chen M. Yang Q. Sun H, et al. Overexpression of FoxO1 causes proliferation of cultured pancreatic beta cells exposed to low nutrition. Biochemistry. 2010;49:218–225. - PubMed
-
- Al-Masri M. Krishnamurthy M. Li J. Fellows GF. Dong HH. Goodyer CG, et al. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia. 2010;53:699–711. - PubMed
-
- Arden KC. FoxOs in tumor suppression and stem cell maintenance. Cell. 2007;128:235–237. - PubMed
-
- Arden KC. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene. 2008;27:2345–2350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

