Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer's disease mice
- PMID: 20610758
- PMCID: PMC6632461
- DOI: 10.1523/JNEUROSCI.0418-10.2010
Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer's disease mice
Abstract
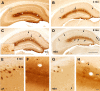
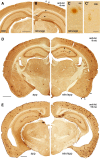
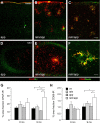
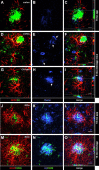
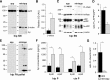
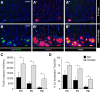
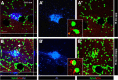
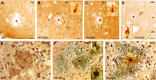
In addition to the fundamental role of the extracellular glycoprotein Reelin in neuronal development and adult synaptic plasticity, alterations in Reelin-mediated signaling have been suggested to contribute to neuronal dysfunction associated with Alzheimer's disease (AD). In vitro data revealed a biochemical link between Reelin-mediated signaling, Tau phosphorylation, and amyloid precursor protein (APP) processing. To directly address the role of Reelin in amyloid-beta plaque and Tau pathology in vivo, we crossed heterozygous Reelin knock-out mice (reeler) with transgenic AD mice to investigate the temporal and spatial AD-like neuropathology. We demonstrate that a reduction in Reelin expression results in enhanced amyloidogenic APP processing, as indicated by the precocious production of amyloid-beta peptides, the significant increase in number and size of amyloid-beta plaques, as well as age-related aggravation of plaque pathology in double mutant compared with single AD mutant mice of both sexes. Numerous amyloid-beta plaques accumulate in the hippocampal formation and neocortex of double mutants, precisely in layers with strongest Reelin expression and highest accumulation of Reelin plaques in aged wild-type mice. Moreover, concentric accumulations of phosphorylated Tau-positive neurons around amyloid-beta plaques were evident in 15-month-old double versus single mutant mice. Silver stainings indicated the presence of neurofibrillary tangles, selectively associated with amyloid-beta plaques and dystrophic neurites in the entorhinal cortex and hippocampus. Our findings suggest that age-related Reelin aggregation and concomitant reduction in Reelin-mediated signaling play a proximal role in synaptic dysfunction associated with amyloid-beta deposition, sufficient to enhance Tau phosphorylation and tangle formation in the hippocampal formation in aged Reelin-deficient transgenic AD mice.
Figures
Similar articles
-
Co-localization of Reelin and proteolytic AbetaPP fragments in hippocampal plaques in aged wild-type mice.J Alzheimers Dis. 2010;19(4):1339-57. doi: 10.3233/JAD-2010-1333. J Alzheimers Dis. 2010. PMID: 20061602
-
Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer's disease.J Neurosci. 2007 Mar 14;27(11):2727-33. doi: 10.1523/JNEUROSCI.3758-06.2007. J Neurosci. 2007. PMID: 17360894 Free PMC article.
-
Age-related accumulation of Reelin in amyloid-like deposits.Neurobiol Aging. 2009 May;30(5):697-716. doi: 10.1016/j.neurobiolaging.2007.08.011. Epub 2007 Sep 27. Neurobiol Aging. 2009. PMID: 17904250
-
The Role of Reelin Signaling in Alzheimer's Disease.Mol Neurobiol. 2016 Oct;53(8):5692-700. doi: 10.1007/s12035-015-9459-9. Epub 2015 Oct 21. Mol Neurobiol. 2016. PMID: 26491027 Review.
-
Reelin links Apolipoprotein E4, Tau, and Amyloid-β in Alzheimer's disease.Ageing Res Rev. 2024 Jul;98:102339. doi: 10.1016/j.arr.2024.102339. Epub 2024 May 14. Ageing Res Rev. 2024. PMID: 38754634 Review.
Cited by
-
The Inflammation-Induced Dysregulation of Reelin Homeostasis Hypothesis of Alzheimer's Disease.J Alzheimers Dis. 2024;100(4):1099-1119. doi: 10.3233/JAD-240088. J Alzheimers Dis. 2024. PMID: 38995785 Free PMC article. Review.
-
Reelin protects against amyloid β toxicity in vivo.Sci Signal. 2015 Jul 7;8(384):ra67. doi: 10.1126/scisignal.aaa6674. Sci Signal. 2015. PMID: 26152694 Free PMC article.
-
Pathophysiological Function of ADAMTS Enzymes on Molecular Mechanism of Alzheimer's Disease.Aging Dis. 2016 Jan 11;7(4):479-90. doi: 10.14336/AD.2016.0111. eCollection 2016 Aug. Aging Dis. 2016. PMID: 27493839 Free PMC article. Review.
-
Alzheimer's genes in microglia: a risk worth investigating.Mol Neurodegener. 2023 Nov 20;18(1):90. doi: 10.1186/s13024-023-00679-4. Mol Neurodegener. 2023. PMID: 37986179 Free PMC article. Review.
-
Characterization of hippocampal Cajal-Retzius cells during development in a mouse model of Alzheimer's disease (Tg2576).Neural Regen Res. 2014 Feb 15;9(4):394-401. doi: 10.4103/1673-5374.128243. Neural Regen Res. 2014. PMID: 25206826 Free PMC article.
References
-
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–49964. - PubMed
-
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. - PubMed
-
- Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, Hammer RE, Herz J. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci. 2006a;26:2041–2052. - PMC - PubMed
-
- Beffert U, Nematollah Farsian F, Masiulis I, Hammer RE, Yoon SO, Giehl KM, Herz J. ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biol. 2006b;16:2446–2452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
