Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2
- PMID: 20610744
- PMCID: PMC6632482
- DOI: 10.1523/JNEUROSCI.1049-10.2010
Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2
Abstract
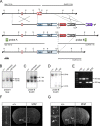
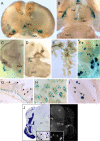
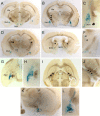
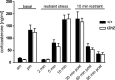
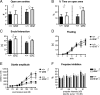
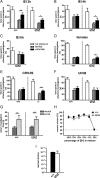
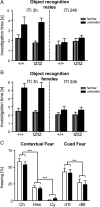
Urocortin 3 (UCN3) is strongly expressed in specific nuclei of the rodent brain, at sites distinct from those expressing urocortin 1 and urocortin 2, the other endogenous ligands of corticotropin-releasing hormone receptor type 2 (CRH-R2). To determine the physiological role of UCN3, we generated UCN3-deficient mice, in which the UCN3 open reading frame was replaced by a tau-lacZ reporter gene. By means of this reporter gene, the nucleus parabrachialis and the premammillary nucleus were identified as previously unknown sites of UCN3 expression. Additionally, the introduced reporter gene enabled the visualization of axonal projections of UCN3-expressing neurons from the superior paraolivary nucleus to the inferior colliculus and from the posterodorsal part of the medial amygdala to the principal nucleus of the bed nucleus of the stria terminalis, respectively. The examination of tau-lacZ reporter gene activity throughout the brain underscored a predominant expression of UCN3 in nuclei functionally connected to the accessory olfactory system. Male and female mice were comprehensively phenotyped but none of the applied tests provided indications for a role of UCN3 in the context of hypothalamic-pituitary-adrenocortical axis regulation, anxiety- or depression-related behavior. However, inspired by the prevalent expression throughout the accessory olfactory system, we identified alterations in social discrimination abilities of male and female UCN3 knock-out mice that were also present in male CRH-R2 knock-out mice. In conclusion, our results suggest a novel role for UCN3 and CRH-R2 related to the processing of social cues and to the establishment of social memories.
Figures

Similar articles
-
Urocortin 2 modulates aspects of social behaviour in mice.Behav Brain Res. 2012 Aug 1;233(2):331-6. doi: 10.1016/j.bbr.2012.05.031. Epub 2012 May 26. Behav Brain Res. 2012. PMID: 22640813
-
Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics.Nat Neurosci. 2016 Nov;19(11):1489-1496. doi: 10.1038/nn.4346. Epub 2016 Jul 18. Nat Neurosci. 2016. PMID: 27428651
-
Expression Patterns of the Neuropeptide Urocortin 3 and Its Receptor CRFR2 in the Mouse Central Auditory System.Front Neural Circuits. 2021 Nov 12;15:747472. doi: 10.3389/fncir.2021.747472. eCollection 2021. Front Neural Circuits. 2021. PMID: 34867212 Free PMC article.
-
The urocortin peptides: biological relevance and laboratory aspects of UCN3 and its receptor.Crit Rev Clin Lab Sci. 2022 Dec;59(8):573-585. doi: 10.1080/10408363.2022.2080175. Epub 2022 Jun 23. Crit Rev Clin Lab Sci. 2022. PMID: 35738909 Review.
-
Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression.Curr Opin Pharmacol. 2002 Feb;2(1):23-33. doi: 10.1016/s1471-4892(01)00117-5. Curr Opin Pharmacol. 2002. PMID: 11786305 Review.
Cited by
-
Conditional Reduction of Adult Born Doublecortin-Positive Neurons Reversibly Impairs Selective Behaviors.Front Behav Neurosci. 2015 Nov 12;9:302. doi: 10.3389/fnbeh.2015.00302. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26617501 Free PMC article.
-
Neural Androgen Receptors Modulate Gene Expression and Social Recognition But Not Social Investigation.Front Behav Neurosci. 2016 Mar 16;10:41. doi: 10.3389/fnbeh.2016.00041. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27014003 Free PMC article.
-
The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response.Curr Mol Pharmacol. 2018;11(1):4-31. doi: 10.2174/1874467210666170302104053. Curr Mol Pharmacol. 2018. PMID: 28260504 Free PMC article. Review.
-
Social isolation of adolescent male rats increases anxiety and K+ -induced dopamine release in the nucleus accumbens: Role of CRF-R1.Eur J Neurosci. 2021 Aug;54(3):4888-4905. doi: 10.1111/ejn.15345. Epub 2021 Jul 7. Eur J Neurosci. 2021. PMID: 34097788 Free PMC article.
-
Neuroimmune connections between corticotropin-releasing hormone and mast cells: novel strategies for the treatment of neurodegenerative diseases.Neural Regen Res. 2021 Nov;16(11):2184-2197. doi: 10.4103/1673-5374.310608. Neural Regen Res. 2021. PMID: 33818491 Free PMC article. Review.
References
-
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. - PubMed
-
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

