HIV infection upregulates caveolin 1 expression to restrict virus production
- PMID: 20610713
- PMCID: PMC2937623
- DOI: 10.1128/JVI.00763-10
HIV infection upregulates caveolin 1 expression to restrict virus production
Abstract
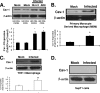
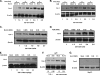
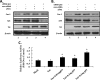
Caveolin 1 (Cav-1) is a major protein of a specific membrane lipid raft known as caveolae. Cav-1 interacts with the gp41 of the human immunodeficiency virus (HIV) envelope, but the role of Cav-1 in HIV replication and pathogenesis is not known. In this report, we demonstrate that HIV infection in primary human monocyte-derived macrophages (MDMs), THP-1 macrophages, and U87-CD4 cells results in a dramatic upregulation of Cav-1 expression mediated by HIV Tat. The activity of p53 is essential for Tat-induced Cav-1 expression, as our findings show enhanced phosphorylation of serine residues at amino acid positions 15 and 46 in the presence of Tat with a resulting Cav-1 upregulation. Furthermore, inhibition of p38 mitogen-activated protein kinase (MAPK) blocked phosphorylation of p53 in the presence of Tat. Infection studies of Cav-1-overexpressing cells reveal a significant reduction of HIV production. Taken together, these results suggest that HIV infection enhances the expression of Cav-1, which subsequently causes virus reduction, suggesting that Cav-1 may contribute to persistent infection in macrophages.
Figures




Similar articles
-
Caveolin 1 inhibits HIV replication by transcriptional repression mediated through NF-κB.J Virol. 2011 Jun;85(11):5483-93. doi: 10.1128/JVI.00254-11. Epub 2011 Mar 23. J Virol. 2011. PMID: 21430048 Free PMC article.
-
p53-derived host restriction of HIV-1 replication by protein kinase R-mediated Tat phosphorylation and inactivation.J Virol. 2015 Apr;89(8):4262-80. doi: 10.1128/JVI.03087-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653431 Free PMC article.
-
HIV and HIV-Tat inhibit LPS-induced IL-27 production in human macrophages by distinct intracellular signaling pathways.J Leukoc Biol. 2017 Sep;102(3):925-939. doi: 10.1189/jlb.4A0716-332RR. Epub 2017 Jul 11. J Leukoc Biol. 2017. PMID: 28698313
-
The Role of Caveolin 1 in HIV Infection and Pathogenesis.Viruses. 2017 May 26;9(6):129. doi: 10.3390/v9060129. Viruses. 2017. PMID: 28587148 Free PMC article. Review.
-
Regulation of human immunodeficiency virus type 1 and cytokine gene expression in myeloid cells by NF-kappa B/Rel transcription factors.Microbiol Rev. 1995 Sep;59(3):481-505. doi: 10.1128/mr.59.3.481-505.1995. Microbiol Rev. 1995. PMID: 7565415 Free PMC article. Review.
Cited by
-
Caveolin-1 suppresses human immunodeficiency virus-1 replication by inhibiting acetylation of NF-κB.Virology. 2012 Oct 10;432(1):110-9. doi: 10.1016/j.virol.2012.05.016. Epub 2012 Jun 28. Virology. 2012. PMID: 22748181 Free PMC article.
-
HIV inhibits endothelial reverse cholesterol transport through impacting subcellular Caveolin-1 trafficking.Retrovirology. 2015 Jul 15;12:62. doi: 10.1186/s12977-015-0188-y. Retrovirology. 2015. PMID: 26169283 Free PMC article.
-
Annexins as organizers of cholesterol- and sphingomyelin-enriched membrane microdomains in Niemann-Pick type C disease.Cell Mol Life Sci. 2012 Jun;69(11):1773-85. doi: 10.1007/s00018-011-0894-0. Epub 2011 Dec 13. Cell Mol Life Sci. 2012. PMID: 22159585 Free PMC article. Review.
-
Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections.Viruses. 2020 Apr 26;12(5):487. doi: 10.3390/v12050487. Viruses. 2020. PMID: 32357558 Free PMC article. Review.
-
The Potential Contribution of Caveolin 1 to HIV Latent Infection.Pathogens. 2020 Oct 27;9(11):896. doi: 10.3390/pathogens9110896. Pathogens. 2020. PMID: 33121153 Free PMC article. Review.
References
-
- Ariumi, Y., A. Kaida, M. Hatanaka, and K. Shimotohno. 2001. Functional cross-talk of HIV-1 Tat with p53 through its C-terminal domain. Biochem. Biophys. Res. Commun. 287:556-561. - PubMed
-
- Bist, A., C. J. Fielding, and P. E. Fielding. 2000. p53 regulates caveolin gene transcription, cell cholesterol, and growth by a novel mechanism. Biochemistry 39:1966-1972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

