Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana
- PMID: 20605913
- PMCID: PMC2938153
- DOI: 10.1104/pp.110.157776
Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana
Abstract
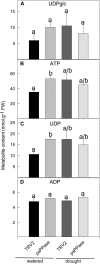
The role of pyrophosphate in primary metabolism is poorly understood. Here, we report on the transient down-regulation of plastid-targeted soluble inorganic pyrophosphatase in Nicotiana benthamiana source leaves. Physiological and metabolic perturbations were particularly evident in chloroplastic central metabolism, which is reliant on fast and efficient pyrophosphate dissipation. Plants lacking plastidial soluble inorganic pyrophosphatase (psPPase) were characterized by increased pyrophosphate levels, decreased starch content, and alterations in chlorophyll and carotenoid biosynthesis, while constituents like amino acids (except for histidine, serine, and tryptophan) and soluble sugars and organic acids (except for malate and citrate) remained invariable from the control. Furthermore, translation of Rubisco was significantly affected, as observed for the amounts of the respective subunits as well as total soluble protein content. These changes were concurrent with the fact that plants with reduced psPPase were unable to assimilate carbon to the same extent as the controls. Furthermore, plants with lowered psPPase exposed to mild drought stress showed a moderate wilting phenotype and reduced vitality, which could be correlated to reduced abscisic acid levels limiting stomatal closure. Taken together, the results suggest that plastidial pyrophosphate dissipation through psPPase is indispensable for vital plant processes.
Figures

Similar articles
-
Inhibition of plastid PPase and NTT leads to major changes in starch and tuber formation in potato.J Exp Bot. 2018 Apr 9;69(8):1913-1924. doi: 10.1093/jxb/ery051. J Exp Bot. 2018. PMID: 29538769 Free PMC article.
-
Overexpression of VP, a vacuolar H+-pyrophosphatase gene in wheat (Triticum aestivum L.), improves tobacco plant growth under Pi and N deprivation, high salinity, and drought.J Exp Bot. 2014 Feb;65(2):683-96. doi: 10.1093/jxb/ert442. J Exp Bot. 2014. Retraction in: J Exp Bot. 2016 Apr;67(9):2913. doi: 10.1093/jxb/erw149 PMID: 24474810 Free PMC article. Retracted.
-
In Nicotiana species, an artificial microRNA corresponding to the virulence modulating region of Potato spindle tuber viroid directs RNA silencing of a soluble inorganic pyrophosphatase gene and the development of abnormal phenotypes.Virology. 2014 Feb;450-451:266-77. doi: 10.1016/j.virol.2013.12.019. Epub 2014 Jan 10. Virology. 2014. PMID: 24503090
-
Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage.J Plant Physiol. 2012 Mar 1;169(4):336-44. doi: 10.1016/j.jplph.2011.10.010. Epub 2011 Dec 3. J Plant Physiol. 2012. PMID: 22137606
-
Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops.Crit Rev Biotechnol. 2021 Aug;41(5):669-691. doi: 10.1080/07388551.2021.1874280. Epub 2021 Feb 1. Crit Rev Biotechnol. 2021. PMID: 33525946 Review.
Cited by
-
Abscisic acid refines the synthesis of chloroplast proteins in maize (Zea mays) in response to drought and light.PLoS One. 2012;7(11):e49500. doi: 10.1371/journal.pone.0049500. Epub 2012 Nov 13. PLoS One. 2012. PMID: 23152915 Free PMC article.
-
A nondiscriminating glutamyl-tRNA synthetase in the plasmodium apicoplast: the first enzyme in an indirect aminoacylation pathway.J Biol Chem. 2013 Nov 8;288(45):32539-32552. doi: 10.1074/jbc.M113.507467. Epub 2013 Sep 26. J Biol Chem. 2013. PMID: 24072705 Free PMC article.
-
Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis.Int J Mol Sci. 2018 Nov 16;19(11):3617. doi: 10.3390/ijms19113617. Int J Mol Sci. 2018. PMID: 30453498 Free PMC article.
-
UDP-Sugar Producing Pyrophosphorylases: Distinct and Essential Enzymes With Overlapping Substrate Specificities, Providing de novo Precursors for Glycosylation Reactions.Front Plant Sci. 2019 Jan 4;9:1822. doi: 10.3389/fpls.2018.01822. eCollection 2018. Front Plant Sci. 2019. PMID: 30662444 Free PMC article. Review.
-
Pyrophosphate levels strongly influence ascorbate and starch content in tomato fruit.Front Plant Sci. 2013 Aug 9;4:308. doi: 10.3389/fpls.2013.00308. eCollection 2013. Front Plant Sci. 2013. PMID: 23950759 Free PMC article.
References
-
- Aharoni A, Jongsma MA, Bouwmeester HJ. (2005) Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci 10: 594–602 - PubMed
-
- Baltscheffsky M, Schultz A, Baltscheffsky H. (1999) H+-PPases: a tightly membrane-bound family. FEBS Lett 457: 527–533 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72: 248–252 - PubMed
-
- Chappell J. (2002) The genetics and molecular genetics of terpene and sterol origami. Curr Opin Plant Biol 5: 151–157 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

