Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety
- PMID: 20605909
- PMCID: PMC2957775
- DOI: 10.1124/jpet.110.170969
Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety
Abstract
Alcoholism and anxiety disorders have a huge impact on society and afflict 17.6 million and 40 million people in the United States, respectively. A strong comorbidity exists between alcoholism and anxiety disorders. Indeed, alcohol withdrawal-induced anxiety is a primary contributing factor for relapse, and anxiolytics are a common adjuvant therapy prescribed for treatment-seeking alcoholics. It is thought that the use of alcohol to self-medicate and relieve anxiety contributes to the development of addiction. Treatment for anxiety disorders and alcoholism exist but are not universally effective. The delta opioid receptor (DOR) plays a role in both alcohol consumption and anxiety, making it a very interesting clinical target. Two pharmacologically distinct DORs have been described: DOR1 and DOR2. We find here that the relative specificity of DOR agonists for DOR1 or DOR2 can greatly affect the effects they exert on ethanol consumption and anxiety. The DOR1 agonist 2-methyl-4aα-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aα-octahydro-quinolino[2,3,30g]isoquinoline (TAN-67), although not effective in decreasing anxiety-like behavior in naive mice, has anxiolytic-like properties in ethanol-withdrawn mice. In contrast, a less subtype-selective agonist, (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80), while also reducing anxiety-like behavior, increases ethanol consumption. In addition, we found that the conical anxiolytic diazepam [DZ; 7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2(1H)-one] is a less effective anxiolytic in ethanol-withdrawn mice than in naive mice. Together, our findings suggest that selective DOR agonists can decrease anxiety-like behavior and are more effective than diazepam at reducing ethanol consumption. We believe the dual efficacy of DOR1 agonists makes these receptors an interesting therapeutic target for treatment-seeking alcoholics.
Figures
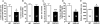
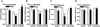
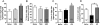
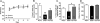
Similar articles
-
Distinctive modulation of ethanol place preference by delta opioid receptor-selective agonists.Drug Alcohol Depend. 2012 Apr 1;122(1-2):156-9. doi: 10.1016/j.drugalcdep.2011.09.024. Epub 2011 Oct 21. Drug Alcohol Depend. 2012. PMID: 22018601 Free PMC article.
-
Involvement of delta opioid receptors in alcohol withdrawal-induced mechanical allodynia in male C57BL/6 mice.Drug Alcohol Depend. 2016 Oct 1;167:190-8. doi: 10.1016/j.drugalcdep.2016.08.017. Epub 2016 Aug 21. Drug Alcohol Depend. 2016. PMID: 27567436 Free PMC article.
-
Dopamine-independent psychostimulant activity of a delta-agonist.Behav Pharmacol. 2008 Mar;19(2):113-9. doi: 10.1097/FBP.0b013e3282f62c1b. Behav Pharmacol. 2008. PMID: 18332675
-
Role of mu and delta opioid receptors in alcohol drinking behaviour.Curr Drug Abuse Rev. 2008 Jun;1(2):239-52. doi: 10.2174/1874473710801020239. Curr Drug Abuse Rev. 2008. PMID: 19630722 Review.
-
Pharmacological traits of delta opioid receptors: pitfalls or opportunities?Psychopharmacology (Berl). 2013 Jul;228(1):1-18. doi: 10.1007/s00213-013-3129-2. Epub 2013 May 7. Psychopharmacology (Berl). 2013. PMID: 23649885 Free PMC article. Review.
Cited by
-
Alcohol Exposure Induces Depressive and Anxiety-like Behaviors via Activating Ferroptosis in Mice.Int J Mol Sci. 2022 Nov 10;23(22):13828. doi: 10.3390/ijms232213828. Int J Mol Sci. 2022. PMID: 36430312 Free PMC article.
-
Alcohol self-administration, anxiety, and cortisol levels predict changes in delta opioid receptor function in the ventral tegmental area.Behav Neurosci. 2012 Aug;126(4):515-22. doi: 10.1037/a0029027. Epub 2012 Jun 18. Behav Neurosci. 2012. PMID: 22708955 Free PMC article.
-
Targeting opioid dysregulation in depression for the development of novel therapeutics.Pharmacol Ther. 2019 Sep;201:51-76. doi: 10.1016/j.pharmthera.2019.04.009. Epub 2019 Apr 30. Pharmacol Ther. 2019. PMID: 31051197 Free PMC article. Review.
-
Chronic Alcohol Drinking Drives Sex-Specific Differences in Affective Behavior and Medial Prefrontal Cortex Activity in CRF1:Cre:tdTomato Transgenic Rats.eNeuro. 2023 Jul 13;10(7):ENEURO.0055-23.2023. doi: 10.1523/ENEURO.0055-23.2023. Print 2023 Jul. eNeuro. 2023. PMID: 37414553 Free PMC article.
-
Receptor expression and signaling properties in the brain, and structural ligand motifs that contribute to delta opioid receptor agonist-induced seizures.Neuropharmacology. 2023 Jul 1;232:109526. doi: 10.1016/j.neuropharm.2023.109526. Epub 2023 Mar 31. Neuropharmacology. 2023. PMID: 37004753 Free PMC article. Review.
References
-
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, et al. (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017 - PubMed
-
- Buonopane A, Petrakis IL. (2005) Pharmacotherapy of alcohol use disorders. Subst Use Misuse 40:2001–2020, 2043–2048 - PubMed
-
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. (2003) Up-regulation and trafficking of δ opioid receptor in a model of chronic inflammation: implications for pain control. Pain 101:199–208 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
