Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats
- PMID: 20600163
- PMCID: PMC2981634
- DOI: 10.1016/j.mvr.2010.06.004
Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats
Abstract
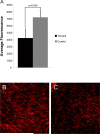
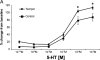
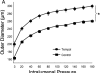
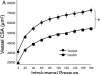
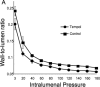
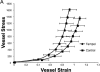
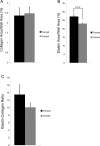
Increased reactive oxygen species (ROS) production is involved in the pathogenesis of hypertension and stroke. The effects of ROS on cerebral vessels from hypertensive rats have not been studied. We hypothesized that tempol, a superoxide dismutase mimetic, would prevent middle cerebral artery (MCA) remodeling in stroke-prone spontaneously hypertensive rats (SHRSP). Six-week-old male SHRSP were treated with tempol (1mM) for 6weeks. The MCA was then removed and mounted in a pressure myograph to study tone generation, vessel reactivity, and passive vessel structure. Data are shown as mean±SEM, tempol vs. control. Plasma thiobarbituric acid reactive substances (TBARS) were decreased by tempol treatment (14.15±1.46 vs. 20.55±1.25nM of malondialdehyde [MDA]/ml, p=0.008). Maximum serotonin-induced constriction was increased by tempol treatment, without changes in dilation to adenosine diphosphate or tone generation. At an intralumenal pressure of 80mmHg, tempol caused a dramatic increase in the MCA lumen diameter (246±5 vs. 207±3μm, p<0.001), outer diameter (281±5 vs. 241±3μm, p<0.001), lumen cross-sectional area, and vessel cross-sectional area. Collagen IV mRNA expressions were increased by 2.4-fold after tempol treatment. These results suggest that ROS are involved in the remodeling of the cerebral vasculature of SHRSP and that ROS scavenging can attenuate this process.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures










Similar articles
-
Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats.Am J Hypertens. 2002 Jan;15(1 Pt 1):78-84. doi: 10.1016/s0895-7061(01)02233-6. Am J Hypertens. 2002. PMID: 11824865
-
Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats.Microvasc Res. 2007 May;73(3):198-205. doi: 10.1016/j.mvr.2006.12.001. Epub 2007 Jan 23. Microvasc Res. 2007. PMID: 17250855 Free PMC article.
-
Effect of tempol and tempol plus catalase on intra-renal haemodynamics in spontaneously hypertensive stroke-prone (SHSP) and Wistar rats.J Physiol Biochem. 2017 May;73(2):207-214. doi: 10.1007/s13105-016-0541-1. Epub 2016 Dec 9. J Physiol Biochem. 2017. PMID: 27933463
-
The effect of tempol on endothelium-dependent vasodilatation and blood pressure.Pharmacol Ther. 2009 May;122(2):109-24. doi: 10.1016/j.pharmthera.2009.02.002. Epub 2009 Mar 5. Pharmacol Ther. 2009. PMID: 19268689 Review.
-
Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: a meta-analysis.J Hypertens. 2015 Jan;33(1):14-23. doi: 10.1097/HJH.0000000000000422. J Hypertens. 2015. PMID: 25380160 Review.
Cited by
-
Increased production of superoxide anion contributes to dysfunction of the arteriovenous fistula.Am J Physiol Renal Physiol. 2012 Dec 15;303(12):F1601-7. doi: 10.1152/ajprenal.00449.2012. Epub 2012 Sep 19. Am J Physiol Renal Physiol. 2012. PMID: 22993073 Free PMC article.
-
Vascular cognitive impairment and dementia.Biochim Biophys Acta. 2016 May;1862(5):860-8. doi: 10.1016/j.bbadis.2015.12.015. Epub 2015 Dec 15. Biochim Biophys Acta. 2016. PMID: 26704177 Free PMC article. Review.
-
Time-induced progressive alteration of kir current in cerebral smooth muscle cells of stroke-prone spontaneously hypertensive rats.Int J Hypertens. 2013;2013:849750. doi: 10.1155/2013/849750. Epub 2013 Apr 23. Int J Hypertens. 2013. PMID: 23710341 Free PMC article.
-
Cerebral microhemorrhages: mechanisms, consequences, and prevention.Am J Physiol Heart Circ Physiol. 2017 Jun 1;312(6):H1128-H1143. doi: 10.1152/ajpheart.00780.2016. Epub 2017 Mar 17. Am J Physiol Heart Circ Physiol. 2017. PMID: 28314762 Free PMC article. Review.
-
The development of hypertension and hyperaldosteronism in a rodent model of life-long obesity.Endocrinology. 2012 Apr;153(4):1764-73. doi: 10.1210/en.2011-1176. Epub 2012 Feb 21. Endocrinology. 2012. PMID: 22355066 Free PMC article.
References
-
- Alberts MJ, Ovbiagele B. Current strategies for ischemic stroke prevention: role of multimodal combination therapies. J Neurol. 2007;254(10):1414–26. - PubMed
-
- Baumbach GL, Ghoneim S. Vascular remodeling in hypertension. Scanning Microsc. 1993;7(1):137–42. discussion 143. - PubMed
-
- Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21(6 Pt 1):816–26. - PubMed
-
- Bonacasa B, Sanchez ML, Rodriguez F, Lopez B, Quesada T, Fenoy FJ, Hernandez I. 2-Methoxyestradiol attenuates hypertension and coronary vascular remodeling in spontaneously hypertensive rats. Maturitas. 2008;61(4):310–6. - PubMed
-
- Briones AM, Rodriguez-Criado N, Hernanz R, Garcia-Redondo AB, Rodrigues-Diez RR, Alonso MJ, Egido J, Ruiz-Ortega M, Salaices M. Atorvastatin prevents angiotensin II-induced vascular remodeling and oxidative stress. Hypertension. 2009;54(1):142–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

