Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research
- PMID: 20591882
- PMCID: PMC2991043
- DOI: 10.1136/jmg.2009.074237
Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research
Erratum in
- J Med Genet. 2011 Mar;48(3):216. Van Krieken, Nicola [corrected to Van Grieken, Nicola C]
Abstract
25-30% of families fulfilling the criteria for hereditary diffuse gastric cancer have germline mutations of the CDH1 (E-cadherin) gene. In light of new data and advancement of technologies, a multidisciplinary workshop was convened to discuss genetic testing, surgery, endoscopy and pathology reporting. The updated recommendations include broadening of CDH1 testing criteria such that: histological confirmation of diffuse gastric criteria is only required for one family member; inclusion of individuals with diffuse gastric cancer before the age of 40 years without a family history; and inclusion of individuals and families with diagnoses of both diffuse gastric cancer (including one before the age of 50 years) and lobular breast cancer. Testing is considered appropriate from the age of consent following counselling and discussion with a multidisciplinary team. In addition to direct sequencing, large genomic rearrangements should be sought. Annual mammography and breast MRI from the age of 35 years is recommended for women due to the increased risk for lobular breast cancer. In mutation positive individuals prophylactic total gastrectomy at a centre of excellence should be strongly considered. Protocolised endoscopic surveillance in centres with endoscopists and pathologists experienced with these patients is recommended for: those opting not to have gastrectomy, those with mutations of undetermined significance, and in those families for whom no germline mutation is yet identified. The systematic histological study of prophylactic gastrectomies almost universally shows pre-invasive lesions including in situ signet ring carcinoma with pagetoid spread of signet ring cells. Expert histopathological confirmation of these early lesions is recommended.
Conflict of interest statement
Figures
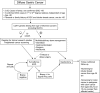



Comment in
-
Hereditary diffuse gastric cancer: lifesaving total gastrectomy for CDH1 mutation carriers.J Med Genet. 2010 Jul;47(7):433-5. doi: 10.1136/jmg.2010.078170. J Med Genet. 2010. PMID: 20591881 No abstract available.
Similar articles
-
Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers.J Med Genet. 2015 Jun;52(6):361-74. doi: 10.1136/jmedgenet-2015-103094. Epub 2015 May 15. J Med Genet. 2015. PMID: 25979631 Free PMC article. Review.
-
Therapeutic and prophylactic gastrectomy in a family with hereditary diffuse gastric cancer secondary to a CDH1 mutation: a case series.World J Surg Oncol. 2018 Jul 14;16(1):143. doi: 10.1186/s12957-018-1415-5. World J Surg Oncol. 2018. PMID: 30007404 Free PMC article.
-
Hereditary gastric cancer.Pathologe. 2012 Nov;33 Suppl 2:231-4. doi: 10.1007/s00292-012-1677-6. Pathologe. 2012. PMID: 23052347 Review.
-
Hereditary diffuse gastric cancer - pathophysiology and clinical management.Best Pract Res Clin Gastroenterol. 2014 Dec;28(6):1055-68. doi: 10.1016/j.bpg.2014.09.007. Epub 2014 Sep 28. Best Pract Res Clin Gastroenterol. 2014. PMID: 25439071 Review.
-
Outcomes of Endoscopic Surveillance in Individuals With Genetic Predisposition to Hereditary Diffuse Gastric Cancer.Gastroenterology. 2019 Jul;157(1):87-96. doi: 10.1053/j.gastro.2019.03.047. Epub 2019 Mar 29. Gastroenterology. 2019. PMID: 30935944
Cited by
-
A novel mutation in the CDH1 gene in a Spanish family with hereditary diffuse gastric cancer.Springerplus. 2016 Jul 26;5(1):1181. doi: 10.1186/s40064-016-2852-7. eCollection 2016. Springerplus. 2016. PMID: 27512640 Free PMC article.
-
Clinical spectrum and pleiotropic nature of CDH1 germline mutations.J Med Genet. 2019 Apr;56(4):199-208. doi: 10.1136/jmedgenet-2018-105807. Epub 2019 Jan 19. J Med Genet. 2019. PMID: 30661051 Free PMC article. Review.
-
Frequency of CDH1, CTNNA1 and CTNND1 Germline Variants in Families with Diffuse and Mixed Gastric Cancer.Cancers (Basel). 2023 Aug 29;15(17):4313. doi: 10.3390/cancers15174313. Cancers (Basel). 2023. PMID: 37686589 Free PMC article.
-
Frequency of CDH1 germline variants and contribution of dietary habits in early age onset gastric cancer patients in Brazil.Gastric Cancer. 2019 Sep;22(5):920-931. doi: 10.1007/s10120-019-00945-9. Epub 2019 Mar 20. Gastric Cancer. 2019. PMID: 30895400 Free PMC article.
-
Sequence-based detection of mutations in cadherin 1 to determine the prevalence of germline mutations in patients with invasive lobular carcinoma of the breast.Hered Cancer Clin Pract. 2014 Jul 19;12(1):17. doi: 10.1186/1897-4287-12-17. eCollection 2014. Hered Cancer Clin Pract. 2014. PMID: 25067988 Free PMC article.
References
-
- Vasen HF, Wijnen JT, Menko FH, Kleibeuker JH, Taal BG, Griffioen G, Nagengast FM, Meijers-Heijboer EH, Bertario L, Varesco L, Bisgaard ML, Mohr J, Fodde R, Khan PM. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 1996;110:1020–7 - PubMed
-
- La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer 1992;70:50–5 - PubMed
-
- Caldas C, Carneiro F, Lynch HT, Yokota J, Wiesner GL, Powell SM, Lewis FR, Huntsman DG, Pharoah PD, Jankowski JA, MacLeod P, Vogelsang H, Keller G, Park KG, Richards FM, Maher ER, Gayther SA, Oliveira C, Grehan N, Wight D, Seruca R, Roviello F, Ponder BA, Jackson CE. Familial gastric cancer: overview and guidelines for management. J Med Genet 1999;36:873–80 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
