The pdh operon is expressed in a subpopulation of stationary-phase bacteria and is important for survival of sugar-starved Streptococcus mutans
- PMID: 20581205
- PMCID: PMC2937364
- DOI: 10.1128/JB.00574-10
The pdh operon is expressed in a subpopulation of stationary-phase bacteria and is important for survival of sugar-starved Streptococcus mutans
Abstract
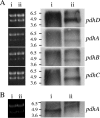
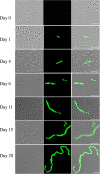
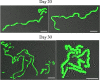
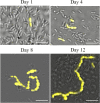
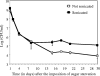
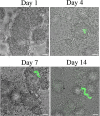
Streptococcus mutans is a facultative member of the oral plaque and is associated with dental caries. It is able to survive long periods of sugar starvation. We show here that inactivation of pdhD, putatively encoding a subunit of the pyruvate dehydrogenase complex, impairs survival of both batch cultures and biofilms. We show that pdhD and the downstream genes pdhA, pdhB, and pdhC form an operon that is predominantly transcribed in stationary phase. Analysis with fluorescent reporters revealed a bimodal expression pattern for the pdh promoter, with less than 1% of stationary-phase populations expressing pdh. When it was first detected, after 1 day of sugar starvation in batch culture, expression was mostly in individual bacteria. At later times, expressing bacteria were often in chains. The lengths of the chains increased with time. We infer that the pdh-expressing subpopulation is able grow and divide and to persist for extended times in stationary phase.
Figures


Similar articles
-
Persistence of Streptococcus mutans in stationary-phase batch cultures and biofilms.Appl Environ Microbiol. 2004 Oct;70(10):6181-7. doi: 10.1128/AEM.70.10.6181-6187.2004. Appl Environ Microbiol. 2004. PMID: 15466565 Free PMC article.
-
The pyruvate dehydrogenase complex of Mycoplasma hyopneumoniae contains a novel lipoyl domain arrangement.Gene. 2003 Nov 13;319:99-106. doi: 10.1016/s0378-1119(03)00798-4. Gene. 2003. PMID: 14597175
-
Role of intracellular polysaccharide in persistence of Streptococcus mutans.J Bacteriol. 2009 Dec;191(23):7315-22. doi: 10.1128/JB.00425-09. Epub 2009 Oct 2. J Bacteriol. 2009. PMID: 19801415 Free PMC article.
-
Guardian genes ensuring subsistence of oral Streptococcus mutans.Crit Rev Microbiol. 2020 Aug;46(4):475-491. doi: 10.1080/1040841X.2020.1796579. Epub 2020 Jul 28. Crit Rev Microbiol. 2020. PMID: 32720594 Review.
-
[Streptococcus mutans and oral streptococci in dental plaque].Can J Microbiol. 2011 Jan;57(1):1-20. doi: 10.1139/w10-095. Can J Microbiol. 2011. PMID: 21217792 Review. French.
Cited by
-
Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) eluate against adhesion and colonization by Streptococcus mutans.Sci Rep. 2018 Mar 22;8(1):5056. doi: 10.1038/s41598-018-23354-x. Sci Rep. 2018. PMID: 29568011 Free PMC article.
-
RNA-Seq Reveals Enhanced Sugar Metabolism in Streptococcus mutans Co-cultured with Candida albicans within Mixed-Species Biofilms.Front Microbiol. 2017 Jun 8;8:1036. doi: 10.3389/fmicb.2017.01036. eCollection 2017. Front Microbiol. 2017. PMID: 28642749 Free PMC article.
-
Small RNA SmsR1 modulates acidogenicity and cariogenic virulence by affecting protein acetylation in Streptococcus mutans.PLoS Pathog. 2024 Apr 15;20(4):e1012147. doi: 10.1371/journal.ppat.1012147. eCollection 2024 Apr. PLoS Pathog. 2024. PMID: 38620039 Free PMC article.
-
Genetic characterization of glyoxalase pathway in oral streptococci and its contribution to interbacterial competition.J Oral Microbiol. 2024 Mar 3;16(1):2322241. doi: 10.1080/20002297.2024.2322241. eCollection 2024. J Oral Microbiol. 2024. PMID: 38440286 Free PMC article.
-
Molecular Basis of Stationary Phase Survival and Applications.Front Microbiol. 2017 Oct 16;8:2000. doi: 10.3389/fmicb.2017.02000. eCollection 2017. Front Microbiol. 2017. PMID: 29085349 Free PMC article. Review.
References
-
- Carlsson, J. 1997. Bacterial metabolism in dental biofilms. Adv. Dent. Res. 11:75-80. - PubMed
-
- Carlsson, J. 1970. Nutritional requirements of Streptococcus mutans. Caries Res. 4:305-320. - PubMed
-
- Chary, V. K., M. Busuioc, J. A. Renye, Jr., and P. J. Piggot. 2005. Vectors that facilitate the replacement of transcriptional lacZ fusions in Streptococcus mutans and Bacillus subtilis with fusions to gfp or gusA. FEMS Microbiol. Lett. 247:171-176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

