Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer
- PMID: 20578891
- PMCID: PMC2935335
- DOI: 10.1089/thy.2010.1646
Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer
Abstract
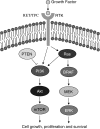
Background: Aberrant activation of the phosphatidylinositol-3 kinase (PI3K)/Akt pathway plays a fundamental role in thyroid tumorigenesis, particularly in follicular thyroid cancer (FTC) and aggressive thyroid cancer, such as anaplastic thyroid cancer (ATC). As the drivers of this process, many genetic alterations activating the PI3K/Akt pathway have been identified in thyroid cancer in recent years.
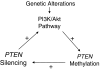
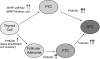
Summary: This review summarizes the current knowledge on major genetic alterations in the PI3K/Akt pathway. These include PIK3CA mutations and genomic amplification/copy gain, Ras mutations, PTEN mutations, RET/PTC and PPARgamma/Pax8 rearrangements, as well as amplification/copy gain of PIK3CB, PDK1, Akt, and various receptor tyrosine kinase genes. Most of these genetic alterations are particularly common in FTC and many of them are even more common in ATC; they are generally less common in papillary thyroid cancer (PTC), in which the MAP kinase (MAPK) pathway activated by the BRAF mutation instead plays a major role. Methylation and, thus, epigenetic silencing of PTEN, a major negative regulator of the PI3K/Akt pathway, occurs in close association with activating genetic alterations of the PI3K/Akt pathway, constituting a unique self-enhancement mechanism for this pathway. Many of these genetic alterations are mutually exclusive in differentiated thyroid tumors, but with increasing concurrence from benign tumors to FTC to ATC. RET/PTC, Ras, and receptor tyrosine kinase could dually activate the PI3K/Akt and MAPK pathways. Most cases of ATC harbor genetic alterations in these genes or other genetic combinations that can activate both pathways. It is proposed that genetic alterations in the PI3K/Akt pathway promote thyroid cell transformation to FTC and that genetic alterations in the MAPK pathway promote cell transformation to PTC; accumulation of multiple genetic alterations that can activate both pathways promotes thyroid cancer aggressiveness and progression to ATC.
Conclusions: Genetic alterations are common in the PI3K/Akt pathway in thyroid cancer and play a fundamental role in the tumorigenesis and progression of this cancer. This provides a strong basis for the emerging development of novel genetic-based diagnostic, prognostic, and therapeutic strategies for thyroid cancer.
Figures
Similar articles
-
Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers.J Clin Endocrinol Metab. 2008 Aug;93(8):3106-16. doi: 10.1210/jc.2008-0273. Epub 2008 May 20. J Clin Endocrinol Metab. 2008. PMID: 18492751
-
Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer.Clin Cancer Res. 2007 Feb 15;13(4):1161-70. doi: 10.1158/1078-0432.CCR-06-1125. Clin Cancer Res. 2007. PMID: 17317825
-
Genetic alterations in the RAS/RAF/mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways in the follicular variant of papillary thyroid carcinoma.Cancer. 2010 Jun 15;116(12):2974-83. doi: 10.1002/cncr.25061. Cancer. 2010. PMID: 20564403
-
A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm.Front Biosci (Landmark Ed). 2011 Jan 1;16(2):422-39. doi: 10.2741/3696. Front Biosci (Landmark Ed). 2011. PMID: 21196179 Review.
-
[Genetic alterations in MAPK and PI3K/Akt signaling pathways and the generation, progression, diagnosis and therapy of thyroid cancer].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012 Dec;29(6):1221-5. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012. PMID: 23469561 Review. Chinese.
Cited by
-
Genetic alterations in anaplastic thyroid carcinoma and targeted therapies.Exp Ther Med. 2019 Oct;18(4):2369-2377. doi: 10.3892/etm.2019.7869. Epub 2019 Aug 8. Exp Ther Med. 2019. PMID: 31555347 Free PMC article. Review.
-
Dysregulation of Glucose Metabolism by Oncogenes and Tumor Suppressors in Cancer Cells.Asian Pac J Cancer Prev. 2018 Sep 26;19(9):2377-2390. doi: 10.22034/APJCP.2018.19.9.2377. Asian Pac J Cancer Prev. 2018. PMID: 30255690 Free PMC article. Review.
-
Monocyte recruitment and activated inflammation are associated with thyroid carcinogenesis in a mouse model.Am J Cancer Res. 2019 Jul 1;9(7):1439-1453. eCollection 2019. Am J Cancer Res. 2019. PMID: 31392080 Free PMC article.
-
Activation of tumor cell proliferation by thyroid hormone in a mouse model of follicular thyroid carcinoma.Oncogene. 2012 Apr 19;31(16):2007-16. doi: 10.1038/onc.2011.390. Epub 2011 Sep 12. Oncogene. 2012. PMID: 21909131 Free PMC article.
-
Synergistic action of a RAF inhibitor and a dual PI3K/mTOR inhibitor in thyroid cancer.Clin Cancer Res. 2011 Oct 15;17(20):6482-9. doi: 10.1158/1078-0432.CCR-11-0933. Epub 2011 Aug 10. Clin Cancer Res. 2011. PMID: 21831957 Free PMC article.
References
-
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. - PubMed
-
- Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. - PubMed
-
- Ciampi R. Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–941. - PubMed
-
- Riesco-Eizaguirre G. Santisteban P. Molecular biology of thyroid cancer initiation. Clin Transl Oncol. 2007;9:686–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

