Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons
- PMID: 20566860
- PMCID: PMC2901465
- DOI: 10.1073/pnas.1005633107
Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons
Abstract
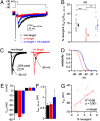
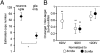
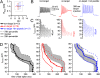
Voltage-gated Na channels in several classes of neurons, including cells of the cerebellum, are subject to an open-channel block and unblock by an endogenous protein. The Na(V)beta4 (Scn4b) subunit is a candidate blocking protein because a free peptide from its cytoplasmic tail, the beta4 peptide, can block open Na channels and induce resurgent current as channels unblock upon repolarization. In heterologous expression systems, however, Na(V)beta4 fails to produce resurgent current. We therefore tested the necessity of this subunit in generating resurgent current, as well as its influence on Na channel gating and action potential firing, by studying cultured cerebellar granule neurons treated with siRNA targeted against Scn4b. Knockdown of Scn4b, confirmed with quantitative RT-PCR, led to five electrophysiological phenotypes: a loss of resurgent current, a reduction of persistent current, a hyperpolarized half-inactivation voltage of transient current, a higher rheobase, and a decrease in repetitive firing. All disruptions of Na currents and firing were rescued by the beta4 peptide. The simplest interpretation is that Na(V)beta4 itself blocks Na channels of granule cells, making this subunit the first blocking protein that is responsible for resurgent current. The results also demonstrate that a known open-channel blocking peptide not only permits a rapid recovery from nonconducting states upon repolarization from positive voltages but also increases Na channel availability at negative potentials by antagonizing fast inactivation. Thus, Na(V)beta4 expression determines multiple aspects of Na channel gating, thereby regulating excitability in cultured cerebellar granule cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cross-species conservation of open-channel block by Na channel β4 peptides reveals structural features required for resurgent Na current.J Neurosci. 2011 Aug 10;31(32):11527-36. doi: 10.1523/JNEUROSCI.1428-11.2011. J Neurosci. 2011. PMID: 21832183 Free PMC article.
-
Antagonism of lidocaine inhibition by open-channel blockers that generate resurgent Na current.J Neurosci. 2013 Mar 13;33(11):4976-87. doi: 10.1523/JNEUROSCI.3026-12.2013. J Neurosci. 2013. PMID: 23486968 Free PMC article.
-
Resurgent Na currents in four classes of neurons of the cerebellum.J Neurophysiol. 2004 Nov;92(5):2831-43. doi: 10.1152/jn.00261.2004. Epub 2004 Jun 22. J Neurophysiol. 2004. PMID: 15212420
-
Resurgent current of voltage-gated Na(+) channels.J Physiol. 2014 Nov 15;592(22):4825-38. doi: 10.1113/jphysiol.2014.277582. Epub 2014 Aug 28. J Physiol. 2014. PMID: 25172941 Free PMC article. Review.
-
The molecular machinery of resurgent sodium current revealed.Neuron. 2005 Jan 20;45(2):185-7. doi: 10.1016/j.neuron.2005.01.006. Neuron. 2005. PMID: 15664169 Review.
Cited by
-
Distinct functional alterations in SCN8A epilepsy mutant channels.J Physiol. 2020 Jan;598(2):381-401. doi: 10.1113/JP278952. Epub 2019 Dec 31. J Physiol. 2020. PMID: 31715021 Free PMC article.
-
Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability.Front Genet. 2013 Oct 28;4:213. doi: 10.3389/fgene.2013.00213. Front Genet. 2013. PMID: 24194747 Free PMC article. Review.
-
More than a pore: ion channel signaling complexes.J Neurosci. 2014 Nov 12;34(46):15159-69. doi: 10.1523/JNEUROSCI.3275-14.2014. J Neurosci. 2014. PMID: 25392484 Free PMC article. Review.
-
GABAA receptors increase excitability and conduction velocity of cerebellar parallel fiber axons.J Neurophysiol. 2012 Jun;107(11):2958-70. doi: 10.1152/jn.01028.2011. Epub 2012 Feb 29. J Neurophysiol. 2012. PMID: 22378171 Free PMC article.
-
The Biophysical Basis Underlying Gating Changes in the p.V1316A Mutant Nav1.7 Channel and the Molecular Pathogenesis of Inherited Erythromelalgia.PLoS Biol. 2016 Sep 21;14(9):e1002561. doi: 10.1371/journal.pbio.1002561. eCollection 2016 Sep. PLoS Biol. 2016. PMID: 27653502 Free PMC article.
References
-
- Oyama F, et al. Sodium channel β4 subunit: Down-regulation and possible involvement in neuritic degeneration in Huntington's disease transgenic mice. J Neurochem. 2006;98:518–529. - PubMed
-
- Miyazaki H, et al. BACE1 modulates filopodia-like protrusions induced by sodium channel β4 subunit. Biochem Biophys Res Commun. 2007;361:43–48. - PubMed
-
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of Na channel β4 as a mechanism for resurgent Na current. Neuron. 2005;45:233–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

