Pyrrole-hyaluronic acid conjugates for decreasing cell binding to metals and conducting polymers
- PMID: 20558330
- PMCID: PMC2942988
- DOI: 10.1016/j.actbio.2010.06.011
Pyrrole-hyaluronic acid conjugates for decreasing cell binding to metals and conducting polymers
Abstract
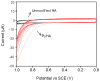
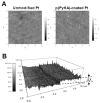
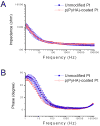
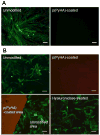
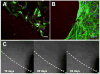
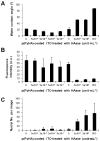
Surface modification of electrically conductive biomaterials has been studied to improve biocompatibility for a number of applications, such as implantable sensors and microelectrode arrays. In this study we electrochemically coated electrodes with biocompatible and non-cell adhesive hyaluronic acid (HA) to reduce cellular adhesion for potential use in neural prostheses. To this end, pyrrole-conjugated hyaluronic acid (PyHA) was synthesized and employed to electrochemically coat platinum, indium-tin oxide and polystyrene sulfonate-doped polypyrrole electrodes. This PyHA conjugate consisted of (1) a pyrrole moiety that allowed the compound to be electrochemically polymerized onto a conductive substrate and (2) non-adhesive HA to minimize cell adhesion and to potentially decrease inflammatory tissue responses. Our characterization results showed the presence of a hydrophilic p(PyHA) layer on the modified electrode, and impedance measurements revealed an impedance that was statistically the same as the unmodified electrode. We found that the p(PyHA)-coated electrodes minimized adhesion and migration of fibroblasts and astrocytes for a minimum of up to 3 months. Also, the coating was stable in physiological solution for 3 months and was stable against enzymatic degradation by hyaluronidase. These studies suggest that this p(PyHA) coating has the potential to be used to mask conducting electrodes from adverse glial responses that occur upon implantation. In addition, electrochemical coating with PyHA could potentially be extended for the surface modification of other metallic and conducting substances, such as stents and biosensors.
Published by Elsevier Ltd.
Figures
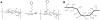
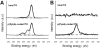

Similar articles
-
Versatile biomimetic conductive polypyrrole films doped with hyaluronic acid of different molecular weights.Acta Biomater. 2018 Oct 15;80:258-268. doi: 10.1016/j.actbio.2018.09.035. Epub 2018 Sep 25. Acta Biomater. 2018. PMID: 30266636
-
Facile and controllable electrochemical fabrication of cell-adhesive polypyrrole electrodes using pyrrole-RGD peptides.Biofabrication. 2017 Nov 14;9(4):045007. doi: 10.1088/1758-5090/aa92a2. Biofabrication. 2017. PMID: 29019465
-
Enzymatically stable, non-cell adhesive, implantable polypyrrole/thiolated hyaluronic acid bioelectrodes for in vivo signal recording.Int J Biol Macromol. 2024 Sep;276(Pt 1):133770. doi: 10.1016/j.ijbiomac.2024.133770. Epub 2024 Jul 9. Int J Biol Macromol. 2024. PMID: 38992547
-
Polypyrrole-based conducting polymers and interactions with biological tissues.J R Soc Interface. 2006 Dec 22;3(11):741-52. doi: 10.1098/rsif.2006.0141. J R Soc Interface. 2006. PMID: 17015302 Free PMC article. Review.
-
Substrate Materials for Biomolecular Immobilization within Electrochemical Biosensors.Biosensors (Basel). 2021 Jul 15;11(7):239. doi: 10.3390/bios11070239. Biosensors (Basel). 2021. PMID: 34356710 Free PMC article. Review.
Cited by
-
Natural bio-based monomers for biomedical applications: a review.Biomater Res. 2021 Apr 1;25(1):8. doi: 10.1186/s40824-021-00208-8. Biomater Res. 2021. PMID: 33795019 Free PMC article. Review.
-
Electrochemical Co-deposition of Polydopamine/Hyaluronic Acid for Anti-biofouling Bioelectrodes.Front Chem. 2019 Apr 30;7:262. doi: 10.3389/fchem.2019.00262. eCollection 2019. Front Chem. 2019. PMID: 31114782 Free PMC article.
-
Amine-functionalized polypyrrole: Inherently cell adhesive conducting polymer.J Biomed Mater Res A. 2015 Jun;103(6):2126-32. doi: 10.1002/jbm.a.35344. Epub 2014 Oct 24. J Biomed Mater Res A. 2015. PMID: 25294089 Free PMC article.
-
Hyaluronic Acid: Redefining Its Role.Cells. 2020 Jul 21;9(7):1743. doi: 10.3390/cells9071743. Cells. 2020. PMID: 32708202 Free PMC article. Review.
-
Electrochemical control of bone microstructure on electroactive surfaces for modulation of stem cells and bone tissue engineering.Sci Technol Adv Mater. 2023 Mar 10;24(1):2183710. doi: 10.1080/14686996.2023.2183710. eCollection 2023. Sci Technol Adv Mater. 2023. PMID: 36926200 Free PMC article.
References
-
- Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants - A review. Prog Mater Sci. 2009;54:397.
-
- Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Progress in Polymer Science. 2007;32:876.
-
- Ratner BD. Biomaterials science : an introduction to materials in medicine. Amsterdam; Boston: Elsevier Academic Press; 2004.
-
- Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius J Anal Chem. 2000;366:611. - PubMed
-
- Uo M, Watari F, Yokoyama A, Matsuno H, Kawasaki T. Tissue reaction around metal implants observed by X-ray scanning analytical microscopy. Biomaterials. 2001;22:677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

