Maternal or infant antiretroviral drugs to reduce HIV-1 transmission
- PMID: 20554982
- PMCID: PMC3440865
- DOI: 10.1056/NEJMoa0911486
Maternal or infant antiretroviral drugs to reduce HIV-1 transmission
Abstract
Background: We evaluated the efficacy of a maternal triple-drug antiretroviral regimen or infant nevirapine prophylaxis for 28 weeks during breast-feeding to reduce postnatal transmission of human immunodeficiency virus type 1 (HIV-1) in Malawi.
Methods: We randomly assigned 2369 HIV-1-positive, breast-feeding mothers with a CD4+ lymphocyte count of at least 250 cells per cubic millimeter and their infants to receive a maternal antiretroviral regimen, infant nevirapine, or no extended postnatal antiretroviral regimen (control group). All mothers and infants received perinatal prophylaxis with single-dose nevirapine and 1 week of zidovudine plus lamivudine. We used the Kaplan-Meier method to estimate the cumulative risk of HIV-1 transmission or death by 28 weeks among infants who were HIV-1-negative 2 weeks after birth. Rates were compared with the use of the log-rank test.
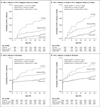
Results: Among mother-infant pairs, 5.0% of infants were HIV-1-positive at 2 weeks of life. The estimated risk of HIV-1 transmission between 2 and 28 weeks was higher in the control group (5.7%) than in either the maternal-regimen group (2.9%, P=0.009) or the infant-regimen group (1.7%, P<0.001). The estimated risk of infant HIV-1 infection or death between 2 and 28 weeks was 7.0% in the control group, 4.1% in the maternal-regimen group (P=0.02), and 2.6% in the infant-regimen group (P<0.001). The proportion of women with neutropenia was higher among those receiving the antiretroviral regimen (6.2%) than among those in either the nevirapine group (2.6%) or the control group (2.3%). Among infants receiving nevirapine, 1.9% had a hypersensitivity reaction.
Conclusions: The use of either a maternal antiretroviral regimen or infant nevirapine for 28 weeks was effective in reducing HIV-1 transmission during breast-feeding. (ClinicalTrials.gov number, NCT00164736.)
2010 Massachusetts Medical Society
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Figures
Comment in
-
Protecting the next generation--eliminating perinatal HIV-1 infection.N Engl J Med. 2010 Jun 17;362(24):2316-8. doi: 10.1056/NEJMe1004406. N Engl J Med. 2010. PMID: 20554987 No abstract available.
-
Maternal or infant antiretroviral drugs to reduce HIV-1 transmission.N Engl J Med. 2010 Nov 11;363(20):1969-70; author reply 1970. doi: 10.1056/NEJMc1009231. N Engl J Med. 2010. PMID: 21067394 No abstract available.
-
Maternal or infant antiretroviral drugs to reduce HIV-1 transmission.N Engl J Med. 2010 Nov 11;363(20):1969; author reply 1970. doi: 10.1056/NEJMc1009231. N Engl J Med. 2010. PMID: 21067395 No abstract available.
-
Maternal or infant antiretroviral drugs to reduce HIV-1 transmission.N Engl J Med. 2010 Nov 11;363(20):1968-9; author reply 1970. doi: 10.1056/NEJMc1009231. N Engl J Med. 2010. PMID: 21067396 No abstract available.
Similar articles
-
Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial.Lancet. 2012 Jun 30;379(9835):2449-2458. doi: 10.1016/S0140-6736(12)60321-3. Epub 2012 Apr 26. Lancet. 2012. PMID: 22541418 Free PMC article. Clinical Trial.
-
Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial.Lancet Infect Dis. 2011 Mar;11(3):171-80. doi: 10.1016/S1473-3099(10)70288-7. Epub 2011 Jan 13. Lancet Infect Dis. 2011. PMID: 21237718 Clinical Trial.
-
Three postpartum antiretroviral regimens to prevent intrapartum HIV infection.N Engl J Med. 2012 Jun 21;366(25):2368-79. doi: 10.1056/NEJMoa1108275. N Engl J Med. 2012. PMID: 22716975 Free PMC article. Clinical Trial.
-
Antiretroviral interventions for preventing breast milk transmission of HIV.Cochrane Database Syst Rev. 2014 Oct 4;2014(10):CD011323. doi: 10.1002/14651858.CD011323. Cochrane Database Syst Rev. 2014. PMID: 25280769 Free PMC article. Review.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394 Review.
Cited by
-
Impact of HIV-1 infection on the feto-maternal crosstalk and consequences for pregnancy outcome and infant health.Semin Immunopathol. 2016 Nov;38(6):727-738. doi: 10.1007/s00281-016-0578-9. Epub 2016 Jul 8. Semin Immunopathol. 2016. PMID: 27392971 Review.
-
Evolving uses of oral reverse transcriptase inhibitors in the HIV-1 epidemic: from treatment to prevention.Retrovirology. 2013 Jul 31;10:82. doi: 10.1186/1742-4690-10-82. Retrovirology. 2013. PMID: 23902855 Free PMC article. Review.
-
Effect of cytomegalovirus infection on breastfeeding transmission of HIV and on the health of infants born to HIV-infected mothers.AIDS. 2015 Apr 24;29(7):831-6. doi: 10.1097/QAD.0000000000000617. AIDS. 2015. PMID: 25985405 Free PMC article. Clinical Trial.
-
Adaptive HIV-specific B cell-derived humoral immune defenses of the intestinal mucosa in children exposed to HIV via breast-feeding.PLoS One. 2013 May 21;8(5):e63408. doi: 10.1371/journal.pone.0063408. Print 2013. PLoS One. 2013. PMID: 23704905 Free PMC article.
-
The case for addressing primary resistance mutations to non-nucleoside reverse transcriptase inhibitors to treat children born from mothers living with HIV in sub-Saharan Africa.J Int AIDS Soc. 2014 Jan 16;17(1):18526. doi: 10.7448/IAS.17.1.18526. eCollection 2014. J Int AIDS Soc. 2014. PMID: 24439027 Free PMC article.
References
-
- Geneva: World Health Organization; 2007. HIV transmission through breastfeeding: a review of available evidence.
-
- Geneva: Joint United Nations Programme on HIV/AIDS; 2007. AIDS epidemic update.
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. - PubMed
-
- Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. - PubMed
-
- WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–455. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
