Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage
- PMID: 20554880
- PMCID: PMC6634595
- DOI: 10.1523/JNEUROSCI.0976-10.2010
Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage
Abstract
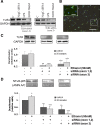
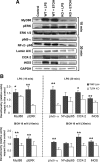
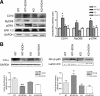
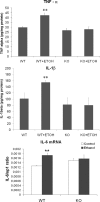
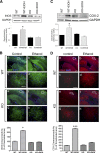
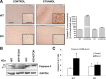
Toll-like receptors play an important role in the innate immune response, although emerging evidence indicates their role in brain injury and neurodegeneration. Alcohol abuse induces brain damage and can sometimes lead to neurodegeneration. We recently found that ethanol can promote TLR4 signaling in glial cells by triggering the induction of inflammatory mediators and causing cell death, suggesting that the TLR4 response could be an important mechanism of ethanol-induced neuroinflammation. This study aims to establish the potential role of TLR4 in both ethanol-induced glial activation and brain damage. Here we report that TLR4 is critical for ethanol-induced inflammatory signaling in glial cells since the knockdown of TLR4, by using both small interfering RNA or cells from TLR4-deficient mice, abolished the activation of microtubule-associated protein kinase and nuclear factor-kappaB pathways and the production of inflammatory mediators by astrocytes. Our results demonstrate, for the first time, that whereas chronic ethanol intake upregulates the immunoreactive levels of CD11b (microglial marker) and glial fibrillary acidic protein (astrocyte marker), and also increases caspase-3 activity and inducible nitric oxide synthase, COX-2, and cytokine levels [interleukin (IL)-1beta, tumor necrosis factor-alpha, IL-6] in the cerebral cortex of female wild-type mice, TLR4 deficiency protects against ethanol-induced glial activation, induction of inflammatory mediators, and apoptosis. Our findings support the critical role of the TLR4 response in the neuroinflammation, brain injury, and possibly in the neurodegeneration induced by chronic ethanol intake.
Figures



Similar articles
-
Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells.J Neurochem. 2013 Jul;126(2):261-73. doi: 10.1111/jnc.12276. Epub 2013 May 8. J Neurochem. 2013. PMID: 23600947
-
Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke.Circulation. 2007 Mar 27;115(12):1599-608. doi: 10.1161/CIRCULATIONAHA.106.603431. Epub 2007 Mar 19. Circulation. 2007. PMID: 17372179
-
Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse.Glia. 2012 May;60(6):948-64. doi: 10.1002/glia.22327. Epub 2012 Mar 19. Glia. 2012. PMID: 22431236
-
Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors.Front Biosci. 2007 Jan 1;12:2616-30. doi: 10.2741/2259. Front Biosci. 2007. PMID: 17127267 Review.
-
Neuroimmune basis of alcoholic brain damage.Int Rev Neurobiol. 2014;118:315-57. doi: 10.1016/B978-0-12-801284-0.00010-5. Int Rev Neurobiol. 2014. PMID: 25175868 Free PMC article. Review.
Cited by
-
Effect of repetitive daily ethanol intoxication on adult rat brain: significant changes in phospholipase A2 enzyme levels in association with increased PARP-1 indicate neuroinflammatory pathway activation.Alcohol. 2013 Feb;47(1):39-45. doi: 10.1016/j.alcohol.2012.09.003. Epub 2012 Oct 25. Alcohol. 2013. PMID: 23102656 Free PMC article.
-
Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia.Glia. 2013 Mar;61(3):349-60. doi: 10.1002/glia.22437. Epub 2012 Oct 25. Glia. 2013. PMID: 23108585 Free PMC article.
-
Neuroimmune mechanisms in fetal alcohol spectrum disorder.Dev Neurobiol. 2012 Oct;72(10):1302-16. doi: 10.1002/dneu.22035. Epub 2012 Sep 1. Dev Neurobiol. 2012. PMID: 22623427 Free PMC article. Review.
-
Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation.PLoS One. 2013;8(3):e59870. doi: 10.1371/journal.pone.0059870. Epub 2013 Mar 29. PLoS One. 2013. PMID: 23555817 Free PMC article.
-
Divergent Roles of APOAI and APOM in the Identification of Alcohol Use Disorder and Their Association With Inflammation and Cognitive Decline: A Pilot Study.Int J Neuropsychopharmacol. 2024 Jul 1;27(7):pyae029. doi: 10.1093/ijnp/pyae029. Int J Neuropsychopharmacol. 2024. PMID: 38970624 Free PMC article.
References
-
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52:448–453. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
