Loss of pRB causes centromere dysfunction and chromosomal instability
- PMID: 20551165
- PMCID: PMC2895196
- DOI: 10.1101/gad.1917310
Loss of pRB causes centromere dysfunction and chromosomal instability
Abstract
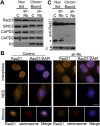
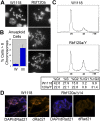
Chromosome instability (CIN) is a common feature of tumor cells. By monitoring chromosome segregation, we show that depletion of the retinoblastoma protein (pRB) causes rates of missegregation comparable with those seen in CIN tumor cells. The retinoblastoma tumor suppressor is frequently inactivated in human cancers and is best known for its regulation of the G1/S-phase transition. Recent studies have shown that pRB inactivation also slows mitotic progression and promotes aneuploidy, but reasons for these phenotypes are not well understood. Here we describe the underlying mitotic defects of pRB-deficient cells that cause chromosome missegregation. Analysis of mitotic cells reveals that pRB depletion compromises centromeric localization of CAP-D3/condensin II and chromosome cohesion, leading to an increase in intercentromeric distance and deformation of centromeric structure. These defects promote merotelic attachment, resulting in failure of chromosome congression and an increased propensity for lagging chromosomes following mitotic delay. While complete loss of centromere function or chromosome cohesion would have catastrophic consequences, these more moderate defects allow pRB-deficient cells to proliferate but undermine the fidelity of mitosis, leading to whole-chromosome gains and losses. These observations explain an important consequence of RB1 inactivation, and suggest that subtle defects in centromere function are a frequent source of merotely and CIN in cancer.
Figures
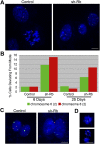
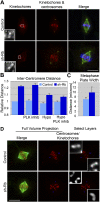
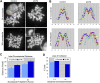
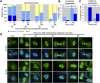

Comment in
-
RB's original CIN?Genes Dev. 2010 Jul 1;24(13):1329-33. doi: 10.1101/gad.1948010. Epub 2010 Jun 15. Genes Dev. 2010. PMID: 20551167 Free PMC article.
-
Genome instability: RB moonlights in mitosis.Nat Rev Mol Cell Biol. 2010 Aug;11(8):541. doi: 10.1038/nrm2951. Nat Rev Mol Cell Biol. 2010. PMID: 20651703 No abstract available.
-
Chromosome instability: RB moonlights in mitosis.Nat Rev Cancer. 2010 Aug;10(8):536. doi: 10.1038/nrc2906. Nat Rev Cancer. 2010. PMID: 20677357 No abstract available.
Similar articles
-
Suppression of genome instability in pRB-deficient cells by enhancement of chromosome cohesion.Mol Cell. 2014 Mar 20;53(6):993-1004. doi: 10.1016/j.molcel.2014.01.032. Epub 2014 Mar 6. Mol Cell. 2014. PMID: 24613344 Free PMC article.
-
Drosophila CAP-D2 is required for condensin complex stability and resolution of sister chromatids.J Cell Sci. 2005 Jun 1;118(Pt 11):2529-43. doi: 10.1242/jcs.02392. J Cell Sci. 2005. PMID: 15923665
-
The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis.Mol Cell Biol. 2005 Oct;25(20):8971-84. doi: 10.1128/MCB.25.20.8971-8984.2005. Mol Cell Biol. 2005. PMID: 16199875 Free PMC article.
-
Condensin in Chromatid Cohesion and Segregation.Cytogenet Genome Res. 2015;147(4):212-6. doi: 10.1159/000444868. Epub 2016 Mar 22. Cytogenet Genome Res. 2015. PMID: 26998746 Review.
-
Centromeric Cohesin: Molecular Glue and Much More.Prog Mol Subcell Biol. 2017;56:485-513. doi: 10.1007/978-3-319-58592-5_20. Prog Mol Subcell Biol. 2017. PMID: 28840250 Review.
Cited by
-
Inactivation of the retinoblastoma gene yields a mouse model of malignant colorectal cancer.Oncogene. 2015 Nov 26;34(48):5890-9. doi: 10.1038/onc.2015.30. Epub 2015 Mar 9. Oncogene. 2015. PMID: 25745996 Free PMC article.
-
Linking abnormal mitosis to the acquisition of DNA damage.J Cell Biol. 2012 Dec 10;199(6):871-81. doi: 10.1083/jcb.201210040. J Cell Biol. 2012. PMID: 23229895 Free PMC article. Review.
-
Hydroxyurea and Caffeine Impact pRb-like Protein-Dependent Chromatin Architecture Profiles in Interphase Cells of Vicia faba.Int J Mol Sci. 2021 Apr 27;22(9):4572. doi: 10.3390/ijms22094572. Int J Mol Sci. 2021. PMID: 33925461 Free PMC article.
-
The multiple connections between pRB and cell metabolism.Curr Opin Cell Biol. 2013 Dec;25(6):735-40. doi: 10.1016/j.ceb.2013.07.012. Epub 2013 Aug 3. Curr Opin Cell Biol. 2013. PMID: 23916769 Free PMC article. Review.
-
Genome maintenance in retinoblastoma: Implications for therapeutic vulnerabilities.Oncol Lett. 2022 Jun;23(6):192. doi: 10.3892/ol.2022.13312. Epub 2022 Apr 29. Oncol Lett. 2022. PMID: 35527780 Free PMC article. Review.
References
-
- Albertson DG, Collins C, McCormick F, Gray JW 2003. Chromosome aberrations in solid tumors. Nat Genet 34: 369–376 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
