SIDL interacts with the dendritic targeting motif of Shal (K(v)4) K+ channels in Drosophila
- PMID: 20550966
- PMCID: PMC3888490
- DOI: 10.1016/j.mcn.2010.06.001
SIDL interacts with the dendritic targeting motif of Shal (K(v)4) K+ channels in Drosophila
Abstract
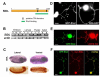
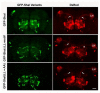
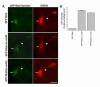
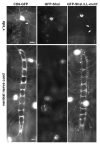
Shal K(+) (K(v)4) channels in mammalian neurons have been shown to be localized exclusively to somato-dendritic regions of neurons, where they function as key determinants of dendritic excitability. To gain insight into the mechanisms underlying dendritic localization of K(v)4 channels, we use Drosophila melanogaster as our model system. We show that Shal K(+) channels display a conserved somato-dendritic localization in vivo in Drosophila. From a yeast-2-hybrid screen, we identify the novel interactor, SIDL (for Shal Interactor of Di-Leucine Motif), as the first target protein reported to bind the highly conserved di-leucine motif (LL-motif) implicated in dendritic targeting. We show that SIDL is expressed primarily in the nervous system, co-localizes with GFP-Shal channels in neurons, and interacts specifically with the LL-motif of Drosophila and mouse Shal channels. We disrupt the Shal-SIDL interaction by mutating the LL-motif on Shal channels, and show that Shal K(+) channels are then mislocalized to some, but not all, axons in vivo. These results suggest that there are multiple mechanisms underlying Shal K(+) channel targeting, one of which depends on the LL-motif. The identification of SIDL may provide the first step for future investigation into the molecular machinery regulating the LL-motif-dependent targeting of K(+) channels.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Shal/K(v)4 channels are required for maintaining excitability during repetitive firing and normal locomotion in Drosophila.PLoS One. 2011 Jan 17;6(1):e16043. doi: 10.1371/journal.pone.0016043. PLoS One. 2011. PMID: 21264215 Free PMC article.
-
An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting.Nat Neurosci. 2003 Mar;6(3):243-50. doi: 10.1038/nn1020. Nat Neurosci. 2003. PMID: 12592409
-
Fast inactivation of Shal (K(v)4) K+ channels is regulated by the novel interactor SKIP3 in Drosophila neurons.Mol Cell Neurosci. 2009 Sep;42(1):33-44. doi: 10.1016/j.mcn.2009.05.003. Epub 2009 May 20. Mol Cell Neurosci. 2009. PMID: 19463952 Free PMC article.
-
Age-related changes in Kv4/Shal and Kv1/Shaker expression in Drosophila and a role for reactive oxygen species.PLoS One. 2021 Dec 21;16(12):e0261087. doi: 10.1371/journal.pone.0261087. eCollection 2021. PLoS One. 2021. PMID: 34932577 Free PMC article.
-
The neuronal Kv4 channel complex.Neurochem Res. 2008 Aug;33(8):1558-67. doi: 10.1007/s11064-008-9650-8. Epub 2008 Mar 21. Neurochem Res. 2008. PMID: 18357523 Free PMC article. Review.
Cited by
-
A Novel Di-Leucine Motif at the N-Terminus of Human Organic Solute Transporter Beta Is Essential for Protein Association and Membrane Localization.PLoS One. 2016 Jun 28;11(6):e0158269. doi: 10.1371/journal.pone.0158269. eCollection 2016. PLoS One. 2016. PMID: 27351185 Free PMC article.
-
The cellular mechanisms that maintain neuronal polarity.Nat Rev Neurosci. 2016 Oct;17(10):611-22. doi: 10.1038/nrn.2016.100. Epub 2016 Aug 11. Nat Rev Neurosci. 2016. PMID: 27511065 Review.
-
Shal/K(v)4 channels are required for maintaining excitability during repetitive firing and normal locomotion in Drosophila.PLoS One. 2011 Jan 17;6(1):e16043. doi: 10.1371/journal.pone.0016043. PLoS One. 2011. PMID: 21264215 Free PMC article.
-
Bilaterian Giant Ankyrins Have a Common Evolutionary Origin and Play a Conserved Role in Patterning the Axon Initial Segment.PLoS Genet. 2016 Dec 2;12(12):e1006457. doi: 10.1371/journal.pgen.1006457. eCollection 2016 Dec. PLoS Genet. 2016. PMID: 27911898 Free PMC article.
-
Control of Sleep Onset by Shal/Kv4 Channels in Drosophila Circadian Neurons.J Neurosci. 2018 Oct 17;38(42):9059-9071. doi: 10.1523/JNEUROSCI.0777-18.2018. Epub 2018 Sep 5. J Neurosci. 2018. PMID: 30185460 Free PMC article.
References
-
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. - PubMed
-
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

