Catalytic reaction mechanism of acetylcholinesterase determined by Born-Oppenheimer ab initio QM/MM molecular dynamics simulations
- PMID: 20550161
- PMCID: PMC2919153
- DOI: 10.1021/jp104258d
Catalytic reaction mechanism of acetylcholinesterase determined by Born-Oppenheimer ab initio QM/MM molecular dynamics simulations
Abstract
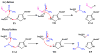
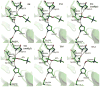
Acetylcholinesterase (AChE) is a remarkably efficient serine hydrolase responsible for the termination of impulse signaling at cholinergic synapses. By employing Born-Oppenheimer molecular dynamics simulations with a B3LYP/6-31G(d) QM/MM potential and the umbrella sampling method, we have characterized its complete catalytic reaction mechanism for hydrolyzing neurotransmitter acetylcholine (ACh) and determined its multistep free-energy reaction profiles for the first time. In both acylation and deacylation reaction stages, the first step involves the nucleophilic attack on the carbonyl carbon, with the triad His447 serving as the general base, and leads to a tetrahedral covalent intermediate stabilized by the oxyanion hole. From the intermediate to the product, the orientation of the His447 ring needs to be adjusted very slightly, and then, the proton transfers from His447 to the product, and the break of the scissile bond happens spontaneously. For the three-pronged oxyanion hole, it only makes two hydrogen bonds with the carbonyl oxygen at either the initial reactant or the final product state, but the third hydrogen bond is formed and stable at all transition and intermediate states during the catalytic process. At the intermediate state of the acylation reaction, a short and low-barrier hydrogen bond (LBHB) is found to be formed between two catalytic triad residues His447 and Glu334, and the spontaneous proton transfer between two residues has been observed. However, it is only about 1-2 kcal/mol stronger than the normal hydrogen bond. In comparison with previous theoretical investigations of the AChE catalytic mechanism, our current study clearly demonstrates the power and advantages of employing Born-Oppenheimer ab initio QM/MM MD simulations in characterizing enzyme reaction mechanisms.
Figures
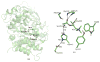

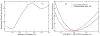
Similar articles
-
Role of the catalytic triad and oxyanion hole in acetylcholinesterase catalysis: an ab initio QM/MM study.J Am Chem Soc. 2002 Sep 4;124(35):10572-7. doi: 10.1021/ja020243m. J Am Chem Soc. 2002. PMID: 12197759
-
Born-Oppenheimer ab initio QM/MM molecular dynamics simulations of the hydrolysis reaction catalyzed by protein arginine deiminase 4.J Phys Chem B. 2009 Dec 31;113(52):16705-10. doi: 10.1021/jp9080614. J Phys Chem B. 2009. PMID: 20028143 Free PMC article.
-
Active site cysteine is protonated in the PAD4 Michaelis complex: evidence from Born-Oppenheimer ab initio QM/MM molecular dynamics simulations.J Phys Chem B. 2009 Sep 24;113(38):12750-8. doi: 10.1021/jp903173c. J Phys Chem B. 2009. PMID: 19507815 Free PMC article.
-
Tutorial Review on the Set-Up and Running of Quantum Mechanical Cluster Models for Enzymatic Reaction Mechanisms.Chemistry. 2024 Oct 28;30(60):e202402468. doi: 10.1002/chem.202402468. Epub 2024 Oct 7. Chemistry. 2024. PMID: 39109881 Review.
-
Unraveling redox pathways of the disulfide bond in dimethyl disulfide: Ab initio modeling.J Mol Model. 2024 May 23;30(6):180. doi: 10.1007/s00894-024-05963-8. J Mol Model. 2024. PMID: 38780881 Review.
Cited by
-
Accumulation of tetrahedral intermediates in cholinesterase catalysis: a secondary isotope effect study.J Am Chem Soc. 2010 Dec 22;132(50):17751-9. doi: 10.1021/ja104496q. Epub 2010 Nov 24. J Am Chem Soc. 2010. PMID: 21105647 Free PMC article.
-
A Comprehensive Review of Cholinesterase Modeling and Simulation.Biomolecules. 2021 Apr 15;11(4):580. doi: 10.3390/biom11040580. Biomolecules. 2021. PMID: 33920972 Free PMC article. Review.
-
Transdermal delivery of resveratrol loaded solid lipid nanoparticle as a microneedle patch: a novel approach for the treatment of Parkinson's disease.Drug Deliv Transl Res. 2025 Mar;15(3):1043-1073. doi: 10.1007/s13346-024-01656-0. Epub 2024 Jun 29. Drug Deliv Transl Res. 2025. PMID: 38949746
-
Structural insights into the putative bacterial acetylcholinesterase ChoE and its substrate inhibition mechanism.J Biol Chem. 2020 Jun 26;295(26):8708-8724. doi: 10.1074/jbc.RA119.011809. Epub 2020 May 5. J Biol Chem. 2020. PMID: 32371400 Free PMC article.
-
The Targeted Pesticides as Acetylcholinesterase Inhibitors: Comprehensive Cross-Organism Molecular Modelling Studies Performed to Anticipate the Pharmacology of Harmfulness to Humans In Vitro.Molecules. 2018 Aug 30;23(9):2192. doi: 10.3390/molecules23092192. Molecules. 2018. PMID: 30200244 Free PMC article.
References
-
- Frey PA, Hegeman AD. Enzymatic Reaction Mechanisms. Oxford University Press; Oxford: 2007.
-
- Kraut DA, Carroll KS, Herschlag D. Annu Rev Biochem. 2003;72:517–571. - PubMed
-
- Potashman MH, Duggan ME. J Med Chem. 2009;52:1231–1246. - PubMed
-
- Robertson JG. Biochemistry. 2005;44:5561–5571. - PubMed
-
- Schramm VL. Accounts Chem Res. 2003;36:588–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

