Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells
- PMID: 20538855
- PMCID: PMC2919003
- DOI: 10.1128/JVI.00599-10
Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells
Abstract
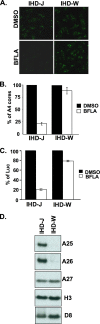
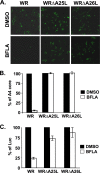
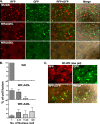
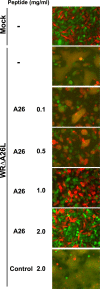
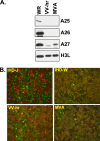
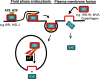
Mature vaccinia virus enters cells through either fluid-phase endocytosis/macropinocytosis or plasma membrane fusion. This may explain the wide range of host cell susceptibilities to vaccinia virus entry; however, it is not known how vaccinia virus chooses between these two pathways and which viral envelope proteins determine such processes. By screening several recombinant viruses and different strains, we found that mature virions containing the vaccinia virus A25 and A26 proteins entered HeLa cells preferentially through a bafilomycin-sensitive entry pathway, whereas virions lacking these two proteins entered through a bafilomycin-resistant pathway. To investigate whether the A25 and A26 proteins contribute to entry pathway specificity, two mutant vaccinia viruses, WRDeltaA25L and WRDeltaA26L, were subsequently generated from the wild-type WR strain. In contrast to the WR strain, both the WRDeltaA25L and WRDeltaA26L viruses became resistant to bafilomycin, suggesting that the removal of the A25 and A26 proteins bypassed the low-pH endosomal requirement for mature virion entry. Indeed, WRDeltaA25L and WRDeltaA26L virus infections of HeLa, CHO-K1, and L cells immediately triggered cell-to-cell fusion at a neutral pH at 1 to 2 h postinfection (p.i.), providing direct evidence that viral fusion machinery is readily activated after the removal of the A25 and A26 proteins to allow virus entry through the plasma membrane. In summary, our data support a model that on vaccinia mature virions, the viral A25 and A26 proteins are low-pH-sensitive fusion suppressors whose inactivation during the endocytic route results in viral and cell membrane fusion. Our results also suggest that during virion morphogenesis, the incorporation of the A25 and A26 proteins into mature virions may help restrain viral fusion activity until the time of infections.
Figures



Similar articles
-
Vaccinia mature virus fusion regulator A26 protein binds to A16 and G9 proteins of the viral entry fusion complex and dissociates from mature virions at low pH.J Virol. 2012 Apr;86(7):3809-18. doi: 10.1128/JVI.06081-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278246 Free PMC article.
-
Vaccinia viral A26 protein is a fusion suppressor of mature virus and triggers membrane fusion through conformational change at low pH.PLoS Pathog. 2019 Jun 20;15(6):e1007826. doi: 10.1371/journal.ppat.1007826. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31220181 Free PMC article.
-
The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components.PLoS Pathog. 2011 Dec;7(12):e1002446. doi: 10.1371/journal.ppat.1002446. Epub 2011 Dec 15. PLoS Pathog. 2011. PMID: 22194690 Free PMC article.
-
Vaccinia virus morphogenesis and dissemination.Trends Microbiol. 2008 Oct;16(10):472-9. doi: 10.1016/j.tim.2008.07.009. Epub 2008 Sep 12. Trends Microbiol. 2008. PMID: 18789694 Review.
-
Poxvirus entry and membrane fusion.Virology. 2006 Jan 5;344(1):48-54. doi: 10.1016/j.virol.2005.09.037. Virology. 2006. PMID: 16364735 Review.
Cited by
-
Membrane fusion during poxvirus entry.Semin Cell Dev Biol. 2016 Dec;60:89-96. doi: 10.1016/j.semcdb.2016.07.015. Epub 2016 Jul 14. Semin Cell Dev Biol. 2016. PMID: 27423915 Free PMC article. Review.
-
Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture.EMBO J. 2011 Jul 26;30(17):3647-61. doi: 10.1038/emboj.2011.245. EMBO J. 2011. PMID: 21792173 Free PMC article.
-
Computational investigations of potential inhibitors of monkeypox virus envelope protein E8 through molecular docking and molecular dynamics simulations.Sci Rep. 2024 Aug 23;14(1):19585. doi: 10.1038/s41598-024-70433-3. Sci Rep. 2024. PMID: 39179615 Free PMC article.
-
Cloak and Dagger: Alternative Immune Evasion and Modulation Strategies of Poxviruses.Viruses. 2015 Aug 21;7(8):4800-25. doi: 10.3390/v7082844. Viruses. 2015. PMID: 26308043 Free PMC article. Review.
-
Single-virus fusion experiments reveal proton influx into vaccinia virions and hemifusion lag times.Biophys J. 2013 Jul 16;105(2):420-31. doi: 10.1016/j.bpj.2013.06.016. Biophys J. 2013. PMID: 23870263 Free PMC article.
References
-
- Armstrong, J. A., D. H. Metz, and M. R. Young. 1973. The mode of entry of vaccinia virus into L cells. J. Gen. Virol. 21:533-537. - PubMed
-
- Carter, G. C., M. Law, M. Hollinshead, and G. L. Smith. 2005. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 86:1279-1290. - PubMed
-
- Chang, A., and D. H. Metz. 1976. Further investigations on the mode of entry of vaccinia virus into cells. J. Gen. Virol. 32:275-282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

