Modulation of endoplasmic reticulum Ca2+ store filling by cyclic ADP-ribose promotes inositol trisphosphate (IP3)-evoked Ca2+ signals
- PMID: 20538594
- PMCID: PMC2915741
- DOI: 10.1074/jbc.M109.095257
Modulation of endoplasmic reticulum Ca2+ store filling by cyclic ADP-ribose promotes inositol trisphosphate (IP3)-evoked Ca2+ signals
Abstract
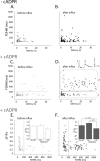
In addition to its well established function in activating Ca(2+) release from the endoplasmic reticulum (ER) through ryanodine receptors (RyR), the second messenger cyclic ADP-ribose (cADPR) also accelerates the activity of SERCA pumps, which sequester Ca(2+) into the ER. Here, we demonstrate a potential physiological role for cADPR in modulating cellular Ca(2+) signals via changes in ER Ca(2+) store content, by imaging Ca(2+) liberation through inositol trisphosphate receptors (IP(3)R) in Xenopus oocytes, which lack RyR. Oocytes were injected with the non-metabolizable analog 3-deaza-cADPR, and cytosolic [Ca(2+)] was transiently elevated by applying voltage-clamp pulses to induce Ca(2+) influx through expressed plasmalemmal nicotinic channels. We observed a subsequent potentiation of global Ca(2+) signals evoked by strong photorelease of IP(3), and increased numbers of local Ca(2+) puffs evoked by weaker photorelease. These effects were not evident with cADPR alone or following cytosolic Ca(2+) elevation alone, indicating that they did not arise through direct actions of cADPR or Ca(2+) on the IP(3)R, but likely resulted from enhanced ER store filling. Moreover, the appearance of a new population of puffs with longer latencies, prolonged durations, and attenuated amplitudes suggests that luminal ER Ca(2+) may modulate IP(3)R function, in addition to simply determining the size of the available store and the electrochemical driving force for release.
Figures

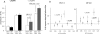
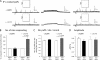
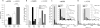

Similar articles
-
Enhanced ER Ca2+ store filling by overexpression of SERCA2b promotes IP3-evoked puffs.Cell Calcium. 2011 Jul;50(1):36-41. doi: 10.1016/j.ceca.2011.04.008. Epub 2011 May 26. Cell Calcium. 2011. PMID: 21616533 Free PMC article.
-
cADPR stimulates SERCA activity in Xenopus oocytes.Cell Calcium. 2009 Mar;45(3):293-9. doi: 10.1016/j.ceca.2008.11.008. Epub 2009 Jan 7. Cell Calcium. 2009. PMID: 19131109 Free PMC article.
-
Two different but converging messenger pathways to intracellular Ca(2+) release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate.EMBO J. 2000 Jun 1;19(11):2549-57. doi: 10.1093/emboj/19.11.2549. EMBO J. 2000. PMID: 10835353 Free PMC article.
-
On a Magical Mystery Tour with 8-Bromo-Cyclic ADP-Ribose: From All-or-None Block to Nanojunctions and the Cell-Wide Web.Molecules. 2020 Oct 16;25(20):4768. doi: 10.3390/molecules25204768. Molecules. 2020. PMID: 33081414 Free PMC article. Review.
-
Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling.Sci China Life Sci. 2011 Aug;54(8):699-711. doi: 10.1007/s11427-011-4197-3. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786193 Review.
Cited by
-
Stochastic simulations of pattern formation in excitable media.PLoS One. 2012;7(8):e42508. doi: 10.1371/journal.pone.0042508. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22900025 Free PMC article.
-
Cyclic adenosine diphosphate ribose activates ryanodine receptors, whereas NAADP activates two-pore domain channels.J Biol Chem. 2011 Mar 18;286(11):9136-40. doi: 10.1074/jbc.M110.202002. Epub 2011 Jan 7. J Biol Chem. 2011. PMID: 21216967 Free PMC article.
-
Intercellular Ca(2+) waves: mechanisms and function.Physiol Rev. 2012 Jul;92(3):1359-92. doi: 10.1152/physrev.00029.2011. Physiol Rev. 2012. PMID: 22811430 Free PMC article. Review.
-
Enhanced ER Ca2+ store filling by overexpression of SERCA2b promotes IP3-evoked puffs.Cell Calcium. 2011 Jul;50(1):36-41. doi: 10.1016/j.ceca.2011.04.008. Epub 2011 May 26. Cell Calcium. 2011. PMID: 21616533 Free PMC article.
-
Inhibition of Sarco-Endoplasmic Reticulum Ca2+ ATPase Extends the Lifespan in C. elegans Worms.Front Pharmacol. 2018 Jun 25;9:669. doi: 10.3389/fphar.2018.00669. eCollection 2018. Front Pharmacol. 2018. PMID: 29988547 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

