Heterologous immunity between viruses
- PMID: 20536568
- PMCID: PMC2917921
- DOI: 10.1111/j.0105-2896.2010.00897.x
Heterologous immunity between viruses
Abstract
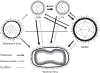
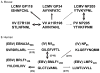
Immune memory responses to previously encountered pathogens can sometimes alter the immune response to and the course of infection of an unrelated pathogen by a process known as heterologous immunity. This response can lead to enhanced or diminished protective immunity and altered immunopathology. Here, we discuss the nature of T-cell cross-reactivity and describe matrices of epitopes from different viruses eliciting cross-reactive CD8(+) T-cell responses. We examine the parameters of heterologous immunity mediated by these cross-reactive T cells during viral infections in mice and humans. We show that heterologous immunity can disrupt T-cell memory pools, alter the complexity of the T-cell repertoire, change patterns of T-cell immunodominance, lead to the selection of viral epitope-escape variants, alter the pathogenesis of viral infections, and, by virtue of the private specificity of T-cell repertoires within individuals, contribute to dramatic variations in viral disease. We propose that heterologous immunity is an important factor in resistance to and variations of human viral infections and that issues of heterologous immunity should be considered in the design of vaccines.
Figures


Similar articles
-
Severity of Acute Infectious Mononucleosis Correlates with Cross-Reactive Influenza CD8 T-Cell Receptor Repertoires.mBio. 2017 Dec 5;8(6):e01841-17. doi: 10.1128/mBio.01841-17. mBio. 2017. PMID: 29208744 Free PMC article.
-
T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens.Nat Immunol. 2002 Jul;3(7):627-34. doi: 10.1038/ni806. Epub 2002 Jun 3. Nat Immunol. 2002. PMID: 12055626
-
Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections.J Immunol. 2003 Apr 15;170(8):4077-86. doi: 10.4049/jimmunol.170.8.4077. J Immunol. 2003. PMID: 12682237
-
CD8 memory T cells: cross-reactivity and heterologous immunity.Semin Immunol. 2004 Oct;16(5):335-47. doi: 10.1016/j.smim.2004.08.014. Semin Immunol. 2004. PMID: 15528078 Free PMC article. Review.
-
The privacy of T cell memory to viruses.Curr Top Microbiol Immunol. 2006;311:117-53. doi: 10.1007/3-540-32636-7_5. Curr Top Microbiol Immunol. 2006. PMID: 17048707 Free PMC article. Review.
Cited by
-
Molecular Mimicry of SARS-CoV-2 Spike Protein in the Nervous System: A Bioinformatics Approach.Comput Struct Biotechnol J. 2022;20:6041-6054. doi: 10.1016/j.csbj.2022.10.022. Epub 2022 Oct 27. Comput Struct Biotechnol J. 2022. PMID: 36317085 Free PMC article.
-
Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future.Viruses. 2012 Oct 29;4(11):2650-69. doi: 10.3390/v4112650. Viruses. 2012. PMID: 23202498 Free PMC article. Review.
-
HLA-DM constrains epitope selection in the human CD4 T cell response to vaccinia virus by favoring the presentation of peptides with longer HLA-DM-mediated half-lives.J Immunol. 2012 Oct 15;189(8):3983-94. doi: 10.4049/jimmunol.1200626. Epub 2012 Sep 10. J Immunol. 2012. PMID: 22966084 Free PMC article.
-
Teicoplanin therapy leading to a significant decrease in viral load in a patient with chronic hepatitis C.J Antimicrob Chemother. 2012 Oct;67(10):2537-8. doi: 10.1093/jac/dks217. Epub 2012 Jun 11. J Antimicrob Chemother. 2012. PMID: 22687891 Free PMC article. No abstract available.
-
The Polarity and Specificity of Antiviral T Lymphocyte Responses Determine Susceptibility to SARS-CoV-2 Infection in Patients with Cancer and Healthy Individuals.Cancer Discov. 2022 Apr 1;12(4):958-983. doi: 10.1158/2159-8290.CD-21-1441. Cancer Discov. 2022. PMID: 35179201 Free PMC article.
References
-
- Clark IA. Heterologous immunity revisited. Parasitology. 2001;122 (Suppl):S51–S59. - PubMed
-
- Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–426. - PubMed
-
- Mackaness GB. The immunological basis of acquired cellular resistance. J Exp Med. 1964;120:105–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI073540/AI/NIAID NIH HHS/United States
- P30 DK032520-26S2/DK/NIDDK NIH HHS/United States
- AI017672/AI/NIAID NIH HHS/United States
- P01 AI049320/AI/NIAID NIH HHS/United States
- R01 AI046578-02/AI/NIAID NIH HHS/United States
- R01 AI017672-20/AI/NIAID NIH HHS/United States
- R37 AI017672/AI/NIAID NIH HHS/United States
- P01 AI046629-010001/AI/NIAID NIH HHS/United States
- P01 AI046629-10A1/AI/NIAID NIH HHS/United States
- R01 AI081675/AI/NIAID NIH HHS/United States
- R01 AI054455/AI/NIAID NIH HHS/United States
- R01 AR035506/AR/NIAMS NIH HHS/United States
- R01 AI081675-24/AI/NIAID NIH HHS/United States
- AI049320/AI/NIAID NIH HHS/United States
- AI073871/AI/NIAID NIH HHS/United States
- U19 AI057330-05S10007/AI/NIAID NIH HHS/United States
- R01 AR035506-13/AR/NIAMS NIH HHS/United States
- U01 AI073871/AI/NIAID NIH HHS/United States
- U01 AI073871-03/AI/NIAID NIH HHS/United States
- P01 AI046629-11/AI/NIAID NIH HHS/United States
- R01 AI046578/AI/NIAID NIH HHS/United States
- U01 AI073871-05S1/AI/NIAID NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- U19 AI057330/AI/NIAID NIH HHS/United States
- P01 AI046629/AI/NIAID NIH HHS/United States
- AI046578/AI/NIAID NIH HHS/United States
- R37 AI017672-25/AI/NIAID NIH HHS/United States
- P01 AI049320-010002/AI/NIAID NIH HHS/United States
- AI46692/AI/NIAID NIH HHS/United States
- AI054455/AI/NIAID NIH HHS/United States
- AI081675/AI/NIAID NIH HHS/United States
- R01 AI054455-01/AI/NIAID NIH HHS/United States
- R01 AI017672/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

