Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats
- PMID: 20534852
- PMCID: PMC6632682
- DOI: 10.1523/JNEUROSCI.5377-09.2010
Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats
Abstract
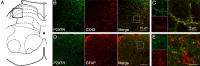
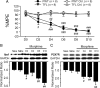
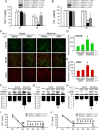
Morphine loses analgesic potency after repeated administration. The underlying mechanism is not fully understood. Glia are thought to be involved in morphine tolerance, and P2X(7) purinergic receptor (P2X(7)R) has been implicated in neuron-glia communication and chronic pain. The present study demonstrated that P2X(7)R immunoreactivity was colocalized with the microglial marker OX42, but not the astrocytic marker GFAP, in the spinal cord. The protein level of spinal P2X(7)R was upregulated after chronic exposure to morphine. Intrathecal administration of Brilliant Blue G (BBG), a selective P2X(7)R inhibitor, significantly attenuated the loss of morphine analgesic potency, P2X(7)R upregulation, and microglial activation. Furthermore, RNA interference targeting the spinal P2X(7)R exhibited a similar tolerance-attenuating effect. Once morphine analgesic tolerance is established, it was no longer affected by intrathecal BBG. Together, our results suggest that spinal P2X(7)R is involved in the induction but not maintenance of morphine tolerance.
Figures

Similar articles
-
Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses.Brain Behav Immun. 2010 Oct;24(7):1176-89. doi: 10.1016/j.bbi.2010.06.001. Epub 2010 Jun 8. Brain Behav Immun. 2010. PMID: 20554014
-
Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: multiple glial-neuronal dialogues in the rat spinal cord.J Pain. 2012 Oct;13(10):945-58. doi: 10.1016/j.jpain.2012.06.007. Epub 2012 Sep 8. J Pain. 2012. PMID: 22968128
-
Site-Specific Regulation of P2X7 Receptor Function in Microglia Gates Morphine Analgesic Tolerance.J Neurosci. 2017 Oct 18;37(42):10154-10172. doi: 10.1523/JNEUROSCI.0852-17.2017. Epub 2017 Sep 18. J Neurosci. 2017. PMID: 28924009 Free PMC article.
-
Roles of P2X7 receptor in glial and neuroblastoma cells: the therapeutic potential of P2X7 receptor antagonists.Mol Neurobiol. 2010 Jun;41(2-3):351-5. doi: 10.1007/s12035-010-8120-x. Epub 2010 Apr 21. Mol Neurobiol. 2010. PMID: 20405342 Review.
-
Regulatory mechanisms and therapeutic potential of microglial inhibitors in neuropathic pain and morphine tolerance.J Zhejiang Univ Sci B. 2020 Mar.;21(3):204-217. doi: 10.1631/jzus.B1900425. J Zhejiang Univ Sci B. 2020. PMID: 32133798 Free PMC article. Review.
Cited by
-
Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential.Nat Rev Neurosci. 2018 Mar;19(3):138-152. doi: 10.1038/nrn.2018.2. Epub 2018 Feb 8. Nat Rev Neurosci. 2018. PMID: 29416128 Review.
-
Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia.J Neuroinflammation. 2016 Mar 1;13(1):53. doi: 10.1186/s12974-016-0520-z. J Neuroinflammation. 2016. PMID: 26931361 Free PMC article.
-
Delay of morphine tolerance by palmitoylethanolamide.Biomed Res Int. 2015;2015:894732. doi: 10.1155/2015/894732. Epub 2015 Mar 22. Biomed Res Int. 2015. PMID: 25874232 Free PMC article.
-
Tissue plasminogen activator contributes to morphine tolerance and induces mechanical allodynia via astrocytic IL-1β and ERK signaling in the spinal cord of mice.Neuroscience. 2013 Sep 5;247:376-85. doi: 10.1016/j.neuroscience.2013.05.018. Epub 2013 May 21. Neuroscience. 2013. PMID: 23707980 Free PMC article.
-
The YTHDF1-TRAF6 pathway regulates the neuroinflammatory response and contributes to morphine tolerance and hyperalgesia in the periaqueductal gray.J Neuroinflammation. 2022 Dec 22;19(1):310. doi: 10.1186/s12974-022-02672-y. J Neuroinflammation. 2022. PMID: 36550542 Free PMC article.
References
-
- Broom DC, Matson DJ, Bradshaw E, Buck ME, Meade R, Coombs S, Matchett M, Ford KK, Yu W, Yuan J, Sun SH, Ochoa R, Krause JE, Wustrow DJ, Cortright DN. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-aminopyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther. 2008;327:620–633. - PubMed
-
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. - PubMed
-
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. - PubMed
-
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
