Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes
- PMID: 20522592
- PMCID: PMC2911061
- DOI: 10.2337/db09-1800
Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes
Abstract
Objective: Our recent study demonstrated that Rac1 and NADPH oxidase activation contributes to cardiomyocyte apoptosis in short-term diabetes. This study was undertaken to investigate if disruption of Rac1 and inhibition of NADPH oxidase would prevent myocardial remodeling in chronic diabetes.
Research design and methods: Diabetes was induced by injection of streptozotocin in mice with cardiomyocyte-specific Rac1 knockout and their wild-type littermates. In a separate experiment, wild-type diabetic mice were treated with vehicle or apocynin in drinking water. Myocardial hypertrophy, fibrosis, endoplasmic reticulum (ER) stress, inflammatory response, and myocardial function were investigated after 2 months of diabetes. Isolated adult rat cardiomyocytes were cultured and stimulated with high glucose.
Results: In diabetic hearts, NADPH oxidase activation, its subunits' expression, and reactive oxygen species production were inhibited by Rac1 knockout or apocynin treatment. Myocardial collagen deposition and cardiomyocyte cross-sectional areas were significantly increased in diabetic mice, which were accompanied by elevated expression of pro-fibrotic genes and hypertrophic genes. Deficiency of Rac1 or apocynin administration reduced myocardial fibrosis and hypertrophy, resulting in improved myocardial function. These effects were associated with a normalization of ER stress markers' expression and inflammatory response in diabetic hearts. In cultured cardiomyocytes, high glucose-induced ER stress was inhibited by blocking Rac1 or NADPH oxidase.
Conclusions: Rac1 via NADPH oxidase activation induces myocardial remodeling and dysfunction in diabetic mice. The role of Rac1 signaling may be associated with ER stress and inflammation. Thus, targeting inhibition of Rac1 and NADPH oxidase may be a therapeutic approach for diabetic cardiomyopathy.
Figures

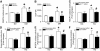
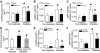




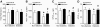
Similar articles
-
Rac1 is required for cardiomyocyte apoptosis during hyperglycemia.Diabetes. 2009 Oct;58(10):2386-95. doi: 10.2337/db08-0617. Epub 2009 Jul 10. Diabetes. 2009. PMID: 19592621 Free PMC article.
-
Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways.Cardiovasc Res. 2013 Jan 1;97(1):77-87. doi: 10.1093/cvr/cvs309. Epub 2012 Oct 1. Cardiovasc Res. 2013. PMID: 23027656
-
Disruption of Rac1 signaling reduces ischemia-reperfusion injury in the diabetic heart by inhibiting calpain.Free Radic Biol Med. 2010 Dec 1;49(11):1804-14. doi: 10.1016/j.freeradbiomed.2010.09.018. Epub 2010 Sep 27. Free Radic Biol Med. 2010. PMID: 20883775
-
Crosstalk of TLR4, vascular NADPH oxidase, and COVID-19 in diabetes: What are the potential implications?Vascul Pharmacol. 2021 Aug;139:106879. doi: 10.1016/j.vph.2021.106879. Epub 2021 May 26. Vascul Pharmacol. 2021. PMID: 34051372 Free PMC article. Review.
-
NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction.Cells. 2021 Jun 22;10(7):1573. doi: 10.3390/cells10071573. Cells. 2021. PMID: 34206537 Free PMC article. Review.
Cited by
-
Fibrosis in Atrial Fibrillation - Role of Reactive Species and MPO.Front Physiol. 2012 Jun 20;3:214. doi: 10.3389/fphys.2012.00214. eCollection 2012. Front Physiol. 2012. PMID: 22723783 Free PMC article.
-
Long-term administration of rosuvastatin prevents contractile and electrical remodelling of diabetic rat heart.J Bioenerg Biomembr. 2013 Aug;45(4):343-52. doi: 10.1007/s10863-013-9514-z. Epub 2013 May 3. J Bioenerg Biomembr. 2013. PMID: 23640692
-
Myocardial remodeling in diabetic cardiomyopathy associated with cardiac mast cell activation.PLoS One. 2013;8(3):e60827. doi: 10.1371/journal.pone.0060827. Epub 2013 Mar 29. PLoS One. 2013. Retraction in: PLoS One. 2024 Jun 11;19(6):e0305584. doi: 10.1371/journal.pone.0305584. PMID: 23556005 Free PMC article. Retracted.
-
Wnt Signaling in Atherosclerosis: Mechanisms to Therapeutic Implications.Biomedicines. 2024 Jan 25;12(2):276. doi: 10.3390/biomedicines12020276. Biomedicines. 2024. PMID: 38397878 Free PMC article. Review.
-
Diabetic cardiomyopathy: pathophysiology and clinical features.Heart Fail Rev. 2013 Mar;18(2):149-66. doi: 10.1007/s10741-012-9313-3. Heart Fail Rev. 2013. PMID: 22453289 Free PMC article. Review.
References
-
- Zarich SW, Nesto RW: Diabetic cardiomyopathy. Am Heart J 1989;118:1000–1012 - PubMed
-
- Cai L, Kang YJ: Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol 2003;3:219–228 - PubMed
-
- Boudina S, Abel ED: Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 - PubMed
-
- Poornima IG, Parikh P, Shannon RP: Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 2006;98:596–605 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

