In vivo regulation of Bcl6 and T follicular helper cell development
- PMID: 20519643
- PMCID: PMC2891136
- DOI: 10.4049/jimmunol.0904023
In vivo regulation of Bcl6 and T follicular helper cell development
Abstract
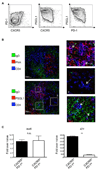
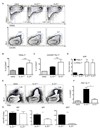
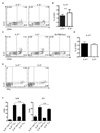
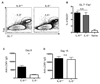
Follicular helper T (T(FH)) cells, defined by expression of the surface markers CXCR5 and programmed death receptor-1 (PD-1) and synthesis of IL-21, require upregulation of the transcriptional repressor Bcl6 for their development and function in B cell maturation in germinal centers. We have explored the role of B cells and the cytokines IL-6 and IL-21 in the in vivo regulation of Bcl6 expression and T(FH) cell development. We found that T(FH) cells are characterized by a Bcl6-dependent downregulation of P-selectin glycoprotein ligand 1 (PSGL1, a CCL19- and CCL21-binding protein), indicating that, like CXCR5 and PD-1 upregulation, modulation of PSGL1 expression is part of the T(FH) cell program of differentiation. B cells were neither required for initial upregulation of Bcl6 nor PSGL1 downregulation, suggesting these events preceded T-B cell interactions, although they were required for full development of the T(FH) cell phenotype, including CXCR5 and PD-1 upregulation, and IL-21 synthesis. Bcl6 upregulation and T(FH) cell differentiation were independent of IL-6 and IL-21, revealing that either cytokine is not absolutely required for development of Bcl6(+) T(FH) cells in vivo. These data increase our understanding of Bcl6 regulation in T(FH) cells and their differentiation in vivo and identifies a new surface marker that may be functionally relevant in this subset.
Figures





Similar articles
-
Cutting Edge: Distinct waves of BCL6 expression during T follicular helper cell development.J Immunol. 2011 Sep 1;187(5):2089-92. doi: 10.4049/jimmunol.1101393. Epub 2011 Jul 29. J Immunol. 2011. PMID: 21804014
-
Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model.J Immunol. 2013 Oct 1;191(7):3705-11. doi: 10.4049/jimmunol.1300378. Epub 2013 Aug 26. J Immunol. 2013. PMID: 23980208 Free PMC article.
-
The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo.J Immunol. 2005 Aug 15;175(4):2340-8. doi: 10.4049/jimmunol.175.4.2340. J Immunol. 2005. PMID: 16081804
-
IL-21 and T follicular helper cells.Int Immunol. 2010 Jan;22(1):7-12. doi: 10.1093/intimm/dxp112. Epub 2009 Nov 23. Int Immunol. 2010. PMID: 19933709 Free PMC article. Review.
-
Follicular helper T cells poise immune responses to the development of autoimmune pathology.Autoimmun Rev. 2011 Apr;10(6):325-30. doi: 10.1016/j.autrev.2010.11.007. Epub 2010 Dec 15. Autoimmun Rev. 2011. PMID: 21167320 Review.
Cited by
-
Inhibition of soluble epoxide hydrolase alleviated atherosclerosis by reducing monocyte infiltration in Ldlr(-/-) mice.J Mol Cell Cardiol. 2016 Sep;98:128-37. doi: 10.1016/j.yjmcc.2016.08.001. Epub 2016 Aug 3. J Mol Cell Cardiol. 2016. PMID: 27496380 Free PMC article.
-
Helper T cell diversity and plasticity.Curr Opin Immunol. 2012 Jun;24(3):297-302. doi: 10.1016/j.coi.2012.01.014. Epub 2012 Feb 15. Curr Opin Immunol. 2012. PMID: 22341735 Free PMC article. Review.
-
TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy.Kidney Int. 2020 Feb;97(2):340-349. doi: 10.1016/j.kint.2019.08.022. Epub 2019 Sep 5. Kidney Int. 2020. PMID: 31748116 Free PMC article.
-
Determination of T Follicular Helper Cell Fate by Dendritic Cells.Front Immunol. 2018 Sep 27;9:2169. doi: 10.3389/fimmu.2018.02169. eCollection 2018. Front Immunol. 2018. PMID: 30319629 Free PMC article. Review.
-
Endosome Traffic Modulates Pro-Inflammatory Signal Transduction in CD4+ T Cells-Implications for the Pathogenesis of Systemic Lupus Erythematosus.Int J Mol Sci. 2023 Jun 28;24(13):10749. doi: 10.3390/ijms241310749. Int J Mol Sci. 2023. PMID: 37445926 Free PMC article. Review.
References
-
- Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. - PubMed
-
- Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of Specific B and T Lymphocyte Interactions in the Lymph Node. Science. 1998;281:96–99. - PubMed
-
- Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

