Uric acid transport and disease
- PMID: 20516647
- PMCID: PMC2877959
- DOI: 10.1172/JCI42344
Uric acid transport and disease
Abstract
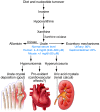
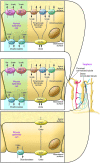
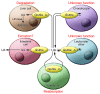
Uric acid is the metabolic end product of purine metabolism in humans. It has antioxidant properties that may be protective but can also be pro-oxidant, depending on its chemical microenvironment. Hyperuricemia predisposes to disease through the formation of urate crystals that cause gout, but hyperuricemia, independent of crystal formation, has also been linked with hypertension, atherosclerosis, insulin resistance, and diabetes. We discuss here the biology of urate metabolism and its role in disease. We also cover the genetics of urate transport, including URAT1, and recent studies identifying SLC2A9, which encodes the glucose transporter family isoform Glut9, as a major determinant of plasma uric acid levels and of gout development.
Figures
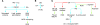
Similar articles
-
GLUT9 influences uric acid concentration in patients with Lesch-Nyhan disease.Int J Rheum Dis. 2018 Jun;21(6):1270-1276. doi: 10.1111/1756-185X.13323. Int J Rheum Dis. 2018. PMID: 29879316
-
Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans.J Biol Chem. 2008 Oct 3;283(40):26834-8. doi: 10.1074/jbc.C800156200. Epub 2008 Aug 13. J Biol Chem. 2008. PMID: 18701466
-
Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate.J Biol Chem. 2010 Nov 5;285(45):35123-32. doi: 10.1074/jbc.M110.121301. Epub 2010 Sep 1. J Biol Chem. 2010. PMID: 20810651 Free PMC article.
-
Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies.Clin Exp Nephrol. 2012 Feb;16(1):89-95. doi: 10.1007/s10157-011-0532-z. Epub 2011 Nov 1. Clin Exp Nephrol. 2012. PMID: 22038265 Review.
-
Renal urate handling: clinical relevance of recent advances.Curr Rheumatol Rep. 2005 Jun;7(3):227-34. doi: 10.1007/s11926-996-0044-0. Curr Rheumatol Rep. 2005. PMID: 15919000 Review.
Cited by
-
Uric acid is an independent risk factor for carotid atherosclerosis in a Japanese elderly population without metabolic syndrome.Cardiovasc Diabetol. 2012 Jan 10;11:2. doi: 10.1186/1475-2840-11-2. Cardiovasc Diabetol. 2012. PMID: 22234039 Free PMC article.
-
Investigation of the Effects and Mechanisms of Dendrobium loddigesii Rolfe Extract on the Treatment of Gout.Evid Based Complement Alternat Med. 2020 Sep 30;2020:4367347. doi: 10.1155/2020/4367347. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33062010 Free PMC article.
-
Global epidemiology of gout: prevalence, incidence and risk factors.Nat Rev Rheumatol. 2015 Nov;11(11):649-62. doi: 10.1038/nrrheum.2015.91. Epub 2015 Jul 7. Nat Rev Rheumatol. 2015. PMID: 26150127 Review.
-
Serum Uric Acid Is Independently Associated with Coronary Calcification in an Asymptomatic Population.J Cardiovasc Transl Res. 2019 Jun;12(3):204-210. doi: 10.1007/s12265-018-9843-8. Epub 2018 Nov 9. J Cardiovasc Transl Res. 2019. PMID: 30414068 Free PMC article.
-
Whole transcriptome expression profiles in kidney samples from rats with hyperuricaemic nephropathy.PLoS One. 2022 Dec 19;17(12):e0276591. doi: 10.1371/journal.pone.0276591. eCollection 2022. PLoS One. 2022. PMID: 36534664 Free PMC article.
References
-
- Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19(5):640–653. - PubMed
-
- Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

