Incorporation of methyl-protonated valine and leucine residues into deuterated ocean pout type III antifreeze protein: expression, crystallization and preliminary neutron diffraction studies
- PMID: 20516595
- PMCID: PMC2882765
- DOI: 10.1107/S1744309110012352
Incorporation of methyl-protonated valine and leucine residues into deuterated ocean pout type III antifreeze protein: expression, crystallization and preliminary neutron diffraction studies
Abstract
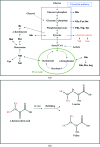
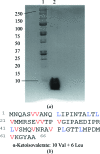
Antifreeze proteins (AFPs) are found in different species from polar, alpine and subarctic regions, where they serve to inhibit ice-crystal growth by adsorption to ice surfaces. Recombinant North Atlantic ocean pout (Macrozoarces americanus) AFP has been used as a model protein to develop protocols for amino-acid-specific hydrogen reverse-labelling of methyl groups in leucine and valine residues using Escherichia coli high-density cell cultures supplemented with the amino-acid precursor alpha-ketoisovalerate. Here, the successful methyl protonation (methyl reverse-labelling) of leucine and valine residues in AFP is reported. Methyl-protonated AFP was expressed in inclusion bodies, refolded in deuterated buffer and purified by cation-exchange chromatography. Crystals were grown in D(2)O buffer by the sitting-drop method. Preliminary neutron Laue diffraction at 293 K using LADI-III at ILL showed in a few 24 h exposures a very low background and clear small spots up to a resolution of 1.80 A from a crystal of dimensions 1.60 x 0.38 x 0.38 mm corresponding to a volume of 0.23 mm(3).
Figures


Similar articles
-
Perdeuteration, purification, crystallization and preliminary neutron diffraction of an ocean pout type III antifreeze protein.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009 Apr 1;65(Pt 4):406-9. doi: 10.1107/S1744309109008574. Epub 2009 Mar 26. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009. PMID: 19342793 Free PMC article.
-
Perdeuteration: improved visualization of solvent structure in neutron macromolecular crystallography.Acta Crystallogr D Biol Crystallogr. 2014 Dec 1;70(Pt 12):3266-72. doi: 10.1107/S1399004714021610. Epub 2014 Nov 28. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 25478844
-
The refined crystal structure of an eel pout type III antifreeze protein RD1 at 0.62-A resolution reveals structural microheterogeneity of protein and solvation.Biophys J. 2003 Feb;84(2 Pt 1):1228-37. doi: 10.1016/S0006-3495(03)74938-8. Biophys J. 2003. PMID: 12547803 Free PMC article.
-
Large crystal growth by thermal control allows combined X-ray and neutron crystallographic studies to elucidate the protonation states in Aspergillus flavus urate oxidase.J R Soc Interface. 2009 Oct 6;6 Suppl 5(Suppl 5):S599-610. doi: 10.1098/rsif.2009.0162.focus. Epub 2009 Jul 8. J R Soc Interface. 2009. PMID: 19586953 Free PMC article. Review.
-
NMR structural studies on antifreeze proteins.Biochem Cell Biol. 1998;76(2-3):284-93. doi: 10.1139/bcb-76-2-3-284. Biochem Cell Biol. 1998. PMID: 9923697 Review.
Cited by
-
Fifteen years of the Protein Crystallography Station: the coming of age of macromolecular neutron crystallography.IUCrJ. 2017 Jan 1;4(Pt 1):72-86. doi: 10.1107/S205225251601664X. eCollection 2017 Jan 1. IUCrJ. 2017. PMID: 28250943 Free PMC article. Review.
-
Peptide backbone circularization enhances antifreeze protein thermostability.Protein Sci. 2017 Oct;26(10):1932-1941. doi: 10.1002/pro.3228. Epub 2017 Jul 25. Protein Sci. 2017. PMID: 28691252 Free PMC article.
References
-
- Antson, A. A., Smith, D. J., Roper, D. I., Lewis, S., Caves, L. S. D., Verma, C. S., Buckley, S. L., Lillford, P. J. & Hubbard, R. E. (2001). J. Mol. Biol.305, 875–889. - PubMed
-
- Artero, J.-B., Härtlein, M., McSweeney, S. & Timmins, P. (2005). Acta Cryst. D61, 1541–1549. - PubMed
-
- Arzt, S., Campbell, J. W., Harding, M. M., Hao, Q. & Helliwell, J. R. (1999). J. Appl. Cryst.32, 554–562.
-
- Blakeley M. P. (2009). Crystallogr. Rev.15, 157–218.
-
- Campbell, J. W., Hao, Q., Harding, M. M., Nguti, N. D. & Wilkinson, C. (1998). J. Appl. Cryst.31, 496–502.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

