Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones
- PMID: 20515451
- PMCID: PMC2890697
- DOI: 10.1186/1743-7075-7-47
Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones
Abstract
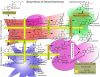
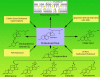
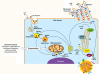
Steroid hormones regulate diverse physiological functions such as reproduction, blood salt balance, maintenance of secondary sexual characteristics, response to stress, neuronal function and various metabolic processes. They are synthesized from cholesterol mainly in the adrenal gland and gonads in response to tissue-specific tropic hormones. These steroidogenic tissues are unique in that they require cholesterol not only for membrane biogenesis, maintenance of membrane fluidity and cell signaling, but also as the starting material for the biosynthesis of steroid hormones. It is not surprising, then, that cells of steroidogenic tissues have evolved with multiple pathways to assure the constant supply of cholesterol needed to maintain optimum steroid synthesis. The cholesterol utilized for steroidogenesis is derived from a combination of sources: 1) de novo synthesis in the endoplasmic reticulum (ER); 2) the mobilization of cholesteryl esters (CEs) stored in lipid droplets through cholesteryl ester hydrolase; 3) plasma lipoprotein-derived CEs obtained by either LDL receptor-mediated endocytic and/or SR-BI-mediated selective uptake; and 4) in some cultured cell systems from plasma membrane-associated free cholesterol. Here, we focus on recent insights into the molecules and cellular processes that mediate the uptake of plasma lipoprotein-derived cholesterol, events connected with the intracellular cholesterol processing and the role of crucial proteins that mediate cholesterol transport to mitochondria for its utilization for steroid hormone production. In particular, we discuss the structure and function of SR-BI, the importance of the selective cholesterol transport pathway in providing cholesterol substrate for steroid biosynthesis and the role of two key proteins, StAR and PBR/TSO in facilitating cholesterol delivery to inner mitochondrial membrane sites, where P450scc (CYP11A) is localized and where the conversion of cholesterol to pregnenolone (the common steroid precursor) takes place.
Figures
Similar articles
-
Steroid hormone synthesis in mitochondria.Mol Cell Endocrinol. 2013 Oct 15;379(1-2):62-73. doi: 10.1016/j.mce.2013.04.014. Epub 2013 Apr 28. Mol Cell Endocrinol. 2013. PMID: 23628605
-
Adrenal cholesterol utilization.Mol Cell Endocrinol. 2007 Feb;265-266:42-5. doi: 10.1016/j.mce.2006.12.001. Epub 2007 Jan 8. Mol Cell Endocrinol. 2007. PMID: 17208360 Review.
-
Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and 'selective' pathway connection.Front Biosci. 2003 Sep 1;8:s998-1029. doi: 10.2741/1165. Front Biosci. 2003. PMID: 12957864 Review.
-
Lipoprotein utilization and cholesterol synthesis by the human fetal adrenal gland.Endocr Rev. 1981 Summer;2(3):306-26. doi: 10.1210/edrv-2-3-306. Endocr Rev. 1981. PMID: 6268397 Review.
-
Regulation of lipid droplets and cholesterol metabolism in adrenal cortical cells.Vitam Horm. 2024;124:79-136. doi: 10.1016/bs.vh.2023.06.007. Epub 2023 Jul 11. Vitam Horm. 2024. PMID: 38408810
Cited by
-
Trafficking of cholesterol from lipid droplets to mitochondria in bovine luteal cells: Acute control of progesterone synthesis.FASEB J. 2020 Aug;34(8):10731-10750. doi: 10.1096/fj.202000671R. Epub 2020 Jul 2. FASEB J. 2020. PMID: 32614098 Free PMC article.
-
HDL biodistribution and brain receptors in zebrafish, using HDLs as vectors for targeting endothelial cells and neural progenitors.Sci Rep. 2021 Mar 19;11(1):6439. doi: 10.1038/s41598-021-85183-9. Sci Rep. 2021. PMID: 33742021 Free PMC article.
-
Effect of Obesity and High-Density Lipoprotein Concentration on the Pathological Characteristics of Alzheimer's Disease in High-Fat Diet-Fed Mice.Int J Mol Sci. 2022 Oct 14;23(20):12296. doi: 10.3390/ijms232012296. Int J Mol Sci. 2022. PMID: 36293147 Free PMC article.
-
The Antitumor Activity of a Lead Thioxanthone is Associated with Alterations in Cholesterol Localization.Molecules. 2018 Dec 12;23(12):3301. doi: 10.3390/molecules23123301. Molecules. 2018. PMID: 30545153 Free PMC article.
-
Effects and Mechanism of Different Phospholipid Diets on Ovary Development in Female Broodstock Pacific White Shrimp, Litopenaeus vannamei.Front Nutr. 2022 Feb 18;9:830934. doi: 10.3389/fnut.2022.830934. eCollection 2022. Front Nutr. 2022. PMID: 35252307 Free PMC article.
References
-
- Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. full_text. - PubMed
-
- Halkersten IDK, Eichorn J, Hechtor O. A requirement for reduced triphosphopyridine nucleotides for cholesterol side-chain cleavage by mitochondrial fractions of bovine adrenal cortex. J Biol Chem. 1961;236:374–380. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

