Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer
- PMID: 20514515
- PMCID: PMC3951776
- DOI: 10.1007/s10549-010-0957-1
Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer
Abstract
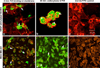
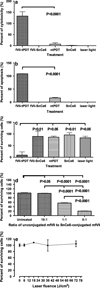
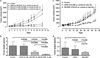
The cell surface receptor tissue factor (TF) is regarded as a common but specific target on angiogenic tumor vascular endothelial cells (VECs) and tumor cells in many types of cancer including breast cancer. The purpose of this study is to develop a selective and effective TF-targeting photodynamic therapy (PDT) by using its natural ligand factor VII (fVII)-conjugated Sn(IV) chlorin e6 (SnCe6) for the treatment of breast cancer. A cross linker EDC was used to covalently conjugate fVII protein to SnCe6, and the binding activity and phototoxicity was confirmed by ELISA and in vitro PDT. The efficacy of fVII-tPDT was assessed in vitro by crystal violet staining assay and in vivo by measuring tumor size in mice carrying murine or human breast cancer xenografts. We show that active site-mutated (K341A) fVII protein can be internalized into breast cancer cells and vascular endothelial growth factor (VEGF)-stimulated human umbilical vein endothelial cells (HUVECs) as angiogenic VECs. fVII-tPDT not only enhances 12-fold the in vitro efficacy but also selectively and effectively kills angiogenic HUVECs and breast cancer cells via specifically binding of fVII to TF and inducing apoptosis and necrosis as the underlying mechanism. Furthermore, fVII-tPDT can significantly inhibit the tumor growth of murine and human breast cancer without obvious toxicities in mice. We conclude that fVII-tPDT using fVII-SnCe6 conjugate can selectively and effectively kill angiogenic VECs and breast cancer cells in vitro and significantly inhibit the tumor growth of murine and human breast cancer in mice.
Figures



Similar articles
-
Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice.BMC Cancer. 2010 May 26;10:235. doi: 10.1186/1471-2407-10-235. BMC Cancer. 2010. PMID: 20504328 Free PMC article.
-
Effective treatment of chemoresistant breast cancer in vitro and in vivo by a factor VII-targeted photodynamic therapy.Br J Cancer. 2011 Apr 26;104(9):1401-9. doi: 10.1038/bjc.2011.88. Epub 2011 Mar 22. Br J Cancer. 2011. PMID: 21427724 Free PMC article.
-
Effective treatment of human lung cancer by targeting tissue factor with a factor VII-targeted photodynamic therapy.Curr Cancer Drug Targets. 2011 Nov;11(9):1069-81. doi: 10.2174/156800911798073023. Curr Cancer Drug Targets. 2011. PMID: 21933104
-
Breast cancer phenotypes regulated by tissue factor-factor VII pathway: Possible therapeutic targets.World J Clin Oncol. 2014 Dec 10;5(5):908-20. doi: 10.5306/wjco.v5.i5.908. World J Clin Oncol. 2014. PMID: 25493229 Free PMC article. Review.
-
Congenital factor VII deficiency: therapy with recombinant activated factor VII -- a critical appraisal.Haemophilia. 2006 Jan;12(1):19-27. doi: 10.1111/j.1365-2516.2006.01180.x. Haemophilia. 2006. PMID: 16409171 Review.
Cited by
-
Factor VII light chain-targeted lidamycin shows intensified therapeutic efficacy for liver cancer.Cancer Biother Radiopharm. 2012 Aug;27(6):384-91. doi: 10.1089/cbr.2012.1209. Epub 2012 May 31. Cancer Biother Radiopharm. 2012. PMID: 22651685 Free PMC article.
-
Tissue factor as a new target for tumor therapy-killing two birds with one stone: a narrative review.Ann Transl Med. 2022 Nov;10(22):1250. doi: 10.21037/atm-22-5067. Ann Transl Med. 2022. PMID: 36544632 Free PMC article. Review.
-
Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems.Pharmaceutics. 2017 Oct 14;9(4):46. doi: 10.3390/pharmaceutics9040046. Pharmaceutics. 2017. PMID: 29036899 Free PMC article. Review.
-
Construction and characterization of a truncated tissue factor‑coagulation‑based composite system for selective thrombosis in tumor blood vessels.Int J Oncol. 2019 Oct;55(4):823-832. doi: 10.3892/ijo.2019.4855. Epub 2019 Aug 12. Int J Oncol. 2019. PMID: 31432158 Free PMC article.
-
Vascular targeting to the SST2 receptor improves the therapeutic response to near-IR two-photon activated PDT for deep-tissue cancer treatment.Biochim Biophys Acta. 2013 Oct;1830(10):4594-603. doi: 10.1016/j.bbagen.2013.05.043. Epub 2013 Jun 7. Biochim Biophys Acta. 2013. PMID: 23747302 Free PMC article.
References
-
- Akhlynina TV, Rosenkranz AA, Jans DA, Sobolev AS. Insulin-mediated intracellular targeting enhances the photodynamic activity of chlorin e6. Cancer Res. 1995;55:1014–1019. - PubMed
-
- Andoh K, Kubota T, Takada M, Tanaka H, Kobayashi N, Maekawa T. Tissue factor activity in leukemia cells. Special reference to disseminated intravascular coagulation. Cancer. 1987;59:748–754. - PubMed
-
- Bauer KA, Conway EM, Bach R, Konigsberg WH, Griffin JD, Demetri G. Tissue factor gene expression in acute myeloblastic leukemia. Thromb Res. 1989;56:425–430. - PubMed
-
- Bevilacqua MP, Gimbrone MA., Jr Inducible endothelial functions in inflammation and coagulation. Semin Thromb He-most. 1987;13:425–433. - PubMed
-
- Birchler M, Viti F, Zardi L, Spiess B, Neri D. Selective targeting and photocoagulation of ocular angiogenesis mediated by a phage-derived human antibody fragment. Nat Biotechnol. 1999;17:984–988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

