Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply
- PMID: 20513425
- PMCID: PMC2896794
- DOI: 10.1016/j.molcel.2010.05.007
Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply
Abstract
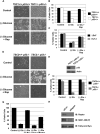
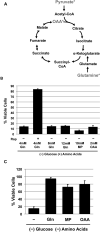
The mTORC1-signaling pathway integrates environmental conditions into distinct signals for cell growth by balancing anabolic and catabolic processes. Accordingly, energetic stress inhibits mTORC1 signaling predominantly through AMPK-dependent activation of TSC1/2. Thus, TSC1/2-/- cells are hypersensitive to glucose deprivation, and this has been linked to increased p53 translation and activation of apoptosis. Herein, we show that mTORC1 inhibition during glucose deprivation prevented not only the execution of death, but also induction of energetic stress. mTORC1 inhibition during glucose deprivation decreased AMPK activation and allowed ATP to remain high, which was both necessary and sufficient for protection. This effect was not due to increased catabolic activities such as autophagy, but rather exclusively due to decreased anabolic processes, reducing energy consumption. Specifically, TSC1/2-/- cells become highly dependent on glutamate dehydrogenase-dependent glutamine metabolism via the TCA cycle for survival. Therefore, mTORC1 inhibition during energetic stress is primarily to balance metabolic demand with supply.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures





Comment in
-
A metabolic (re-)balancing act.Mol Cell. 2010 May 28;38(4):481-2. doi: 10.1016/j.molcel.2010.05.008. Mol Cell. 2010. PMID: 20513422
Similar articles
-
Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex.Mol Cell. 2013 Jan 10;49(1):172-85. doi: 10.1016/j.molcel.2012.10.003. Epub 2012 Nov 8. Mol Cell. 2013. PMID: 23142078 Free PMC article.
-
mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3beta inhibition.Mol Cell Biol. 2009 Sep;29(18):5136-47. doi: 10.1128/MCB.01946-08. Epub 2009 Jul 20. Mol Cell Biol. 2009. PMID: 19620286 Free PMC article.
-
Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors.Cancer Res. 2009 Aug 1;69(15):6107-14. doi: 10.1158/0008-5472.CAN-09-0975. Epub 2009 Jul 14. Cancer Res. 2009. PMID: 19602587 Free PMC article.
-
The TSC1-TSC2 complex: a molecular switchboard controlling cell growth.Biochem J. 2008 Jun 1;412(2):179-90. doi: 10.1042/BJ20080281. Biochem J. 2008. PMID: 18466115 Free PMC article. Review.
-
A complex interplay between Akt, TSC2 and the two mTOR complexes.Biochem Soc Trans. 2009 Feb;37(Pt 1):217-22. doi: 10.1042/BST0370217. Biochem Soc Trans. 2009. PMID: 19143635 Free PMC article. Review.
Cited by
-
Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death.Mol Syst Biol. 2012 Jun 26;8:589. doi: 10.1038/msb.2012.20. Mol Syst Biol. 2012. PMID: 22735335 Free PMC article.
-
A nexus for cellular homeostasis: the interplay between metabolic and signal transduction pathways.Curr Opin Biotechnol. 2015 Aug;34:110-7. doi: 10.1016/j.copbio.2014.12.007. Epub 2015 Jan 3. Curr Opin Biotechnol. 2015. PMID: 25562138 Free PMC article. Review.
-
Continuous sensing of nutrients and growth factors by the mTORC1-TFEB axis.Elife. 2023 Sep 12;12:e74903. doi: 10.7554/eLife.74903. Elife. 2023. PMID: 37698461 Free PMC article.
-
Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling.Science. 2011 Jun 10;332(6035):1322-6. doi: 10.1126/science.1199484. Science. 2011. PMID: 21659605 Free PMC article.
-
LKB1 is a central regulator of tumor initiation and pro-growth metabolism in ErbB2-mediated breast cancer.Cancer Metab. 2013 Aug 14;1(1):18. doi: 10.1186/2049-3002-1-18. Cancer Metab. 2013. PMID: 24280377 Free PMC article.
References
-
- Buzzai M, Bauer DE, Jones RG, Deberardins RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

