Nicotine inhibits Fc epsilon RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through alpha 7/alpha 9/alpha 10-nicotinic receptors
- PMID: 20505147
- PMCID: PMC2954495
- DOI: 10.4049/jimmunol.0902227
Nicotine inhibits Fc epsilon RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through alpha 7/alpha 9/alpha 10-nicotinic receptors
Abstract
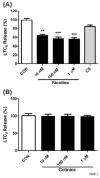
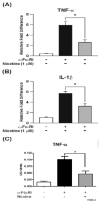
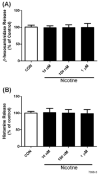
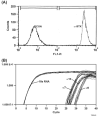
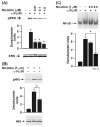
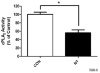
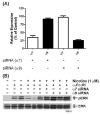
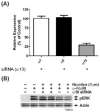
Smokers are less likely to develop some inflammatory and allergic diseases. In Brown-Norway rats, nicotine inhibits several parameters of allergic asthma, including the production of Th2 cytokines and the cysteinyl leukotriene LTC(4). Cysteinyl leukotrienes are primarily produced by mast cells, and these cells play a central role in allergic asthma. Mast cells express a high-affinity receptor for IgE (FcepsilonRI). Following its cross-linking, cells degranulate and release preformed inflammatory mediators (early phase) and synthesize and secrete cytokines/chemokines and leukotrienes (late phase). The mechanism by which nicotine modulates mast cell activation is unclear. Using alpha-bungarotoxin binding and quantitative PCR and PCR product sequencing, we showed that the rat mast/basophil cell line RBL-2H3 expresses nicotinic acetylcholine receptors (nAChRs) alpha7, alpha9, and alpha10; exposure to exceedingly low concentrations of nicotine (nanomolar), but not the biologically inactive metabolite cotinine, for > or = 8 h suppressed the late phase (leukotriene/cytokine production) but not degranulation (histamine and hexosaminidase release). These effects were unrelated to those of nicotine on intracellular free calcium concentration but were causally associated with the inhibition of cytosolic phospholipase A(2) activity and the PI3K/ERK/NF-kappaB pathway, including phosphorylation of Akt and ERK and nuclear translocation of NF-kappaB. The suppressive effect of nicotine on the late-phase response was blocked by the alpha7/alpha9-nAChR antagonists methyllycaconitine and alpha-bungarotoxin, as well as by small interfering RNA knockdown of alpha7-, alpha9-, or alpha10-nAChRs, suggesting a functional interaction between alpha7-, alpha9-, and alpha10-nAChRs that might explain the response of RBL cells to nanomolar concentrations of nicotine. This "hybrid" receptor might serve as a target for novel antiallergic/antiasthmatic therapies.
Figures

Similar articles
-
Rac and protein kinase C-delta regulate ERKs and cytosolic phospholipase A2 in FcepsilonRI signaling to cysteinyl leukotriene synthesis in mast cells.J Immunol. 2004 Jul 1;173(1):624-31. doi: 10.4049/jimmunol.173.1.624. J Immunol. 2004. PMID: 15210825
-
FcepsilonRI-alpha siRNA inhibits the antigen-induced activation of mast cells.Iran J Allergy Asthma Immunol. 2009 Dec;8(4):177-83. Iran J Allergy Asthma Immunol. 2009. PMID: 20404387
-
The Bcl10-Malt1 complex segregates Fc epsilon RI-mediated nuclear factor kappa B activation and cytokine production from mast cell degranulation.J Exp Med. 2006 Feb 20;203(2):337-47. doi: 10.1084/jem.20051982. Epub 2006 Jan 23. J Exp Med. 2006. PMID: 16432253 Free PMC article.
-
Molecular targets on mast cells and basophils for novel therapies.J Allergy Clin Immunol. 2014 Sep;134(3):530-44. doi: 10.1016/j.jaci.2014.03.007. Epub 2014 Apr 24. J Allergy Clin Immunol. 2014. PMID: 24767877 Review.
-
Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a.Immunol Lett. 2010 Jan 18;128(1):36-45. doi: 10.1016/j.imlet.2009.10.007. Epub 2009 Nov 4. Immunol Lett. 2010. PMID: 19895849 Free PMC article. Review.
Cited by
-
Cholinergic Modulation of Type 2 Immune Responses.Front Immunol. 2017 Dec 19;8:1873. doi: 10.3389/fimmu.2017.01873. eCollection 2017. Front Immunol. 2017. PMID: 29312347 Free PMC article. Review.
-
Stimulation of nAchRα7 Receptor Inhibits TNF Synthesis and Secretion in Response to LPS Treatment of Mast Cells by Targeting ERK1/2 and TACE Activation.J Neuroimmune Pharmacol. 2018 Mar;13(1):39-52. doi: 10.1007/s11481-017-9760-7. Epub 2017 Aug 18. J Neuroimmune Pharmacol. 2018. PMID: 28822039
-
Social Networking of Group Two Innate Lymphoid Cells in Allergy and Asthma.Front Immunol. 2018 Nov 20;9:2694. doi: 10.3389/fimmu.2018.02694. eCollection 2018. Front Immunol. 2018. PMID: 30524437 Free PMC article. Review.
-
Altered Intestinal Permeability and Drug Repositioning in a Post-operative Ileus Guinea Pig Model.J Neurogastroenterol Motil. 2021 Oct 30;27(4):639-649. doi: 10.5056/jnm21018. J Neurogastroenterol Motil. 2021. PMID: 34642285 Free PMC article.
-
New Pathways for the Skin's Stress Response: The Cholinergic Neuropeptide SLURP-1 Can Activate Mast Cells and Alter Cytokine Production in Mice.Front Immunol. 2021 Mar 18;12:631881. doi: 10.3389/fimmu.2021.631881. eCollection 2021. Front Immunol. 2021. PMID: 33815383 Free PMC article.
References
-
- Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. J Allergy Clin Iimmunol. 1999;103:1–10. - PubMed
-
- Beasley R, Crane J, Lai CK, Pearce N. Prevalence and etiology of asthma. J Allergy Clin Immunol. 2000;105:S466–472. - PubMed
-
- Wuthrich B. Epidemiology of the allergic diseases: are they really on the increase? Int Arch Allergy Appl Immunol. 1989;90(Suppl 1):3–10. - PubMed
-
- von Mutius E. The rising trends in asthma and allergic disease. Clin Exp Allergy. 1998;28(Suppl 5):45–49. discussion 50-41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

