Effect of hypernatremia on injury caused by energy deficiency: role of T-type Ca2+ channel
- PMID: 20505041
- PMCID: PMC2928636
- DOI: 10.1152/ajpcell.00362.2009
Effect of hypernatremia on injury caused by energy deficiency: role of T-type Ca2+ channel
Abstract
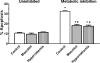
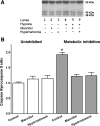
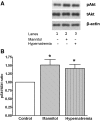
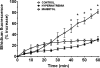
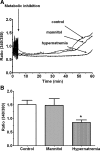
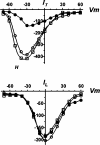
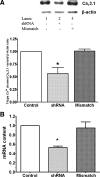
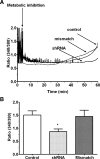
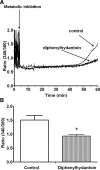
Hypernatremia exerts multiple cellular effects, many of which could influence the outcome of an ischemic event. To further evaluate these effects of hypernatremia, isolated neonatal cardiomyocytes were chronically incubated with medium containing either normal (142 mM) or elevated sodium (167 mM) and then transferred to medium containing deoxyglucose and the electron transport chain inhibitor amobarbital. Chronic hypernatremia diminished the degree of calcium accumulation and reactive oxygen species generation during the period of metabolic inhibition. The improvement in calcium homeostasis was traced in part to the downregulation of the Ca(V)3.1 T-type calcium channel, as deficiency in the Ca(V)3.1 subtype using short hairpin RNA or treatment with an inhibitor of the Ca(V)3.1 variant of the T-type calcium channel (i.e., diphenylhydantoin) attenuated energy deficiency-mediated calcium accumulation and cell death. Although hyperosmotically stressed cells (exposed to 50 mM mannitol) had no effect on T-type calcium channel activity, they were also resistant to death during metabolic inhibition. Both hyperosmotic stress and hypernatremia activated Akt, suggesting that they initiate the phosphatidylinositol 3-kinase/Akt cytoprotective pathway, which protects the cell against calcium overload and oxidative stress. Thus hypernatremia appears to protect the cell against metabolic inhibition by promoting the downregulation of the T-type calcium channel and stimulating cytoprotective protein kinase pathways.
Figures



Similar articles
-
Telmisartan, an angiotensin II type 1 receptor antagonist, attenuates T-type Ca2+ channel expression in neonatal rat cardiomyocytes.Eur J Pharmacol. 2009 May 1;609(1-3):105-12. doi: 10.1016/j.ejphar.2009.03.024. Epub 2009 Mar 16. Eur J Pharmacol. 2009. PMID: 19292980
-
T-type calcium channels are regulated by hypoxia/reoxygenation in ventricular myocytes.Am J Physiol Heart Circ Physiol. 2009 Oct;297(4):H1304-13. doi: 10.1152/ajpheart.00528.2009. Epub 2009 Aug 7. Am J Physiol Heart Circ Physiol. 2009. PMID: 19666840 Free PMC article.
-
17 beta-estradiol modulates expression of low-voltage-activated Ca(V)3.2 T-type calcium channel via extracellularly regulated kinase pathway in cardiomyocytes.Endocrinology. 2009 Feb;150(2):879-88. doi: 10.1210/en.2008-0645. Epub 2008 Oct 1. Endocrinology. 2009. PMID: 18832095
-
Role of the T-type calcium channel CaV3.2 in the chronotropic action of corticosteroids in isolated rat ventricular myocytes.Endocrinology. 2009 Aug;150(8):3726-34. doi: 10.1210/en.2008-1727. Epub 2009 May 14. Endocrinology. 2009. PMID: 19443576
-
Cardiac T-type Ca(2+) channels in the heart.J Mol Cell Cardiol. 2010 Jan;48(1):65-70. doi: 10.1016/j.yjmcc.2009.08.021. Epub 2009 Aug 31. J Mol Cell Cardiol. 2010. PMID: 19729018 Review.
References
-
- Allo SN, Bagby L, Schaffer SW. Taurine depletion, a novel mechanism for cardioprotection from regional ischemia. Am J Physiol Heart Circ Physiol 273: H1956–H1961, 1997 - PubMed
-
- Bhardwaj A, Harukuni I, Murphy SJ, Alkayed NJ, Crain BJ, Koehler RC, Hurn PD, Traystman RJ. Hypertonic saline worsens infarct volume after transient focal ischemia in rats. Stroke 31: 1694–1701, 2000 - PubMed
-
- Costa ADT, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res 77: 344–352, 2008 - PubMed
-
- Fearon IM, Randall AD, Perez-Reyes E, Peers C. Modulation of recombinant T-type Ca2+ channels by hypoxia and glutathione. Pflügers Arch 441: 181–188, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

