Respiratory syncytial virus engineered to express the cystic fibrosis transmembrane conductance regulator corrects the bioelectric phenotype of human cystic fibrosis airway epithelium in vitro
- PMID: 20504917
- PMCID: PMC2897634
- DOI: 10.1128/JVI.00346-10
Respiratory syncytial virus engineered to express the cystic fibrosis transmembrane conductance regulator corrects the bioelectric phenotype of human cystic fibrosis airway epithelium in vitro
Abstract
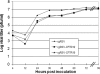
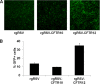
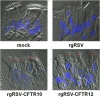
Cystic fibrosis (CF) is the most common lethal recessive genetic disease in the Caucasian population. It is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that is normally expressed in ciliated airway epithelial cells and the submucosal glands of the lung. Since the CFTR gene was first characterized in 1989, a major goal has been to develop an effective gene therapy for CF lung disease, which has the potential to ameliorate morbidity and mortality. Respiratory syncytial virus (RSV) naturally infects the ciliated cells in the human airway epithelium. In addition, the immune response mounted against an RSV infection does not prevent subsequent infections, suggesting that an RSV-based vector might be effectively readministered. To test whether the large 4.5-kb CFTR gene could be expressed by a recombinant RSV and whether infectious virus could be used to deliver CFTR to ciliated airway epithelium derived from CF patients, we inserted the CFTR gene into four sites in a recombinant green fluorescent protein-expressing RSV (rgRSV) genome to generate virus expressing four different levels of CFTR protein. Two of these four rgRSV-CFTR vectors were capable of expressing CFTR with little effect on viral replication. rgRSV-CFTR infection of primary human airway epithelial cultures derived from CF patients resulted in expression of CFTR protein that was properly localized at the luminal surface and corrected the chloride ion channel defect in these cells.
Figures


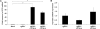

Similar articles
-
CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium.PLoS Biol. 2009 Jul;7(7):e1000155. doi: 10.1371/journal.pbio.1000155. Epub 2009 Jul 21. PLoS Biol. 2009. PMID: 19621064 Free PMC article.
-
Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium.Mol Ther. 2009 Dec;17(12):2067-77. doi: 10.1038/mt.2009.155. Epub 2009 Jul 14. Mol Ther. 2009. PMID: 19603002 Free PMC article.
-
Spliceosome-mediated RNA trans-splicing with recombinant adeno-associated virus partially restores cystic fibrosis transmembrane conductance regulator function to polarized human cystic fibrosis airway epithelial cells.Hum Gene Ther. 2005 Sep;16(9):1116-23. doi: 10.1089/hum.2005.16.1116. Hum Gene Ther. 2005. PMID: 16149910
-
Gene therapy for the respiratory manifestations of cystic fibrosis.Am J Respir Crit Care Med. 1995 Mar;151(3 Pt 2):S75-87. doi: 10.1164/ajrccm/151.3_Pt_2.S75. Am J Respir Crit Care Med. 1995. PMID: 7533609 Review.
-
Personalized medicine in CF: from modulator development to therapy for cystic fibrosis patients with rare CFTR mutations.Am J Physiol Lung Cell Mol Physiol. 2018 Apr 1;314(4):L529-L543. doi: 10.1152/ajplung.00465.2017. Epub 2017 Dec 14. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29351449 Free PMC article. Review.
Cited by
-
Genetic therapies for cystic fibrosis lung disease.Hum Mol Genet. 2011 Apr 15;20(R1):R79-86. doi: 10.1093/hmg/ddr104. Epub 2011 Mar 21. Hum Mol Genet. 2011. PMID: 21422098 Free PMC article. Review.
-
Efficient and Robust Paramyxoviridae Reverse Genetics Systems.mSphere. 2017 Mar 29;2(2):e00376-16. doi: 10.1128/mSphere.00376-16. eCollection 2017 Mar-Apr. mSphere. 2017. PMID: 28405630 Free PMC article.
-
Respiratory syncytial virus-neutralizing serum antibody titers in infants following palivizumab prophylaxis with an abbreviated dosing regimen.PLoS One. 2017 Apr 24;12(4):e0176152. doi: 10.1371/journal.pone.0176152. eCollection 2017. PLoS One. 2017. PMID: 28437470 Free PMC article.
-
Nanotechnology approaches for inhalation treatment of fibrosis.J Drug Target. 2013 Dec;21(10):914-25. doi: 10.3109/1061186X.2013.829078. Epub 2013 Aug 27. J Drug Target. 2013. PMID: 23978292 Free PMC article. Review.
-
Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies.J Virol. 2020 Sep 29;94(20):e01068-20. doi: 10.1128/JVI.01068-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32759319 Free PMC article.
References
-
- Altschuler, M., R. Tritz, and A. Hampel. 1992. A method for generating transcripts with defined 5′ and 3′ termini by autolytic processing. Gene 122:85-90. - PubMed
-
- Amaral, M. D. 2005. Processing of CFTR: traversing the cellular maze—how much CFTR needs to go through to avoid cystic fibrosis? Pediatr. Pulmonol. 39:479-491. - PubMed
-
- Apolonia, L., S. N. Waddington, C. Fernandes, N. J. Ward, G. Bouma, M. P. Blundell, A. J. Thrasher, M. K. Collins, and N. J. Philpott. 2007. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 15:1947-1954. - PubMed
-
- Baatz, J. E., Y. Zou, and T. R. Korfhagen. 2001. Inhibitory effects of tumor necrosis factor-alpha on cationic lipid-mediated gene delivery to airway cells in vitro. Biochim. Biophys. Acta 1535:100-109. - PubMed
-
- Banasik, M. B., and P. B. McCray, Jr. 2010. Integrase-defective lentiviral vectors: progress and applications. Gene Ther. 17:150-157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

