Integrin alpha5beta1 function is regulated by XGIPC/kermit2 mediated endocytosis during Xenopus laevis gastrulation
- PMID: 20498857
- PMCID: PMC2871791
- DOI: 10.1371/journal.pone.0010665
Integrin alpha5beta1 function is regulated by XGIPC/kermit2 mediated endocytosis during Xenopus laevis gastrulation
Erratum in
-
Correction: Integrin α5β1 Function Is Regulated by XGIPC/kermit2 Mediated Endocytosis during Xenopus laevis Gastrulation.PLoS One. 2015 Nov 25;10(11):e0143904. doi: 10.1371/journal.pone.0143904. eCollection 2015. PLoS One. 2015. PMID: 26605543 Free PMC article. No abstract available.
Abstract
During Xenopus gastrulation alpha5beta1 integrin function is modulated in a temporally and spatially restricted manner, however, the regulatory mechanisms behind this regulation remain uncharacterized. Here we report that XGIPC/kermit2 binds to the cytoplasmic domain of the alpha5 subunit and regulates the activity of alpha5beta1 integrin. The interaction of kermit2 with alpha5beta1 is essential for fibronectin (FN) matrix assembly during the early stages of gastrulation. We further demonstrate that kermit2 regulates alpha5beta1 integrin endocytosis downstream of activin signaling. Inhibition of kermit2 function impairs cell migration but not adhesion to FN substrates indicating that integrin recycling is essential for mesoderm cell migration. Furthermore, we find that the alpha5beta1 integrin is colocalized with kermit2 and Rab 21 in embryonic and XTC cells. These data support a model where region specific mesoderm induction acts through kermit2 to regulate the temporally and spatially restricted changes in adhesive properties of the alpha5beta1 integrin through receptor endocytosis.
Conflict of interest statement
Figures
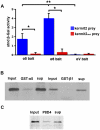





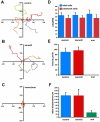
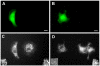
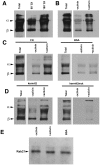

Similar articles
-
PACSIN2 regulates cell adhesion during gastrulation in Xenopus laevis.Dev Biol. 2008 Jul 1;319(1):86-99. doi: 10.1016/j.ydbio.2008.04.007. Epub 2008 May 21. Dev Biol. 2008. PMID: 18495106 Free PMC article.
-
Hes6 is required for MyoD induction during gastrulation.Dev Biol. 2007 Dec 1;312(1):61-76. doi: 10.1016/j.ydbio.2007.09.011. Epub 2007 Sep 16. Dev Biol. 2007. PMID: 17950722
-
Involvement of the eukaryotic initiation factor 6 and kermit2/gipc2 in Xenopus laevis pronephros formation.Int J Dev Biol. 2012;56(5):357-62. doi: 10.1387/ijdb.120009nd. Int J Dev Biol. 2012. PMID: 22689378
-
Cell migration in the Xenopus gastrula.Wiley Interdiscip Rev Dev Biol. 2018 Nov;7(6):e325. doi: 10.1002/wdev.325. Epub 2018 Jun 26. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29944210 Review.
-
Convergent extension in the amphibian, Xenopus laevis.Curr Top Dev Biol. 2020;136:271-317. doi: 10.1016/bs.ctdb.2019.11.013. Epub 2019 Dec 27. Curr Top Dev Biol. 2020. PMID: 31959291 Free PMC article. Review.
Cited by
-
Integrin trafficking in cells and tissues.Nat Cell Biol. 2019 Feb;21(2):122-132. doi: 10.1038/s41556-018-0223-z. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602723 Free PMC article. Review.
-
SH3BP4 promotes neuropilin-1 and α5-integrin endocytosis and is inhibited by Akt.Dev Cell. 2021 Apr 19;56(8):1164-1181.e12. doi: 10.1016/j.devcel.2021.03.009. Epub 2021 Mar 23. Dev Cell. 2021. PMID: 33761321 Free PMC article.
-
Possible Role of Activin in the Adiponectin Paradox-Induced Progress of Alzheimer's Disease.J Alzheimers Dis. 2021;81(2):451-458. doi: 10.3233/JAD-210206. J Alzheimers Dis. 2021. PMID: 33814453 Free PMC article. Review.
-
Integrin trafficking at a glance.J Cell Sci. 2012 Aug 15;125(Pt 16):3695-701. doi: 10.1242/jcs.095810. J Cell Sci. 2012. PMID: 23027580 Free PMC article. Review. No abstract available.
-
GAIP interacting protein C-terminus regulates autophagy and exosome biogenesis of pancreatic cancer through metabolic pathways.PLoS One. 2014 Dec 3;9(12):e114409. doi: 10.1371/journal.pone.0114409. eCollection 2014. PLoS One. 2014. PMID: 25469510 Free PMC article.
References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Pellinen T, Ivaska J. Integrin traffic. J Cell Sci. 2006;119:3723–3731. - PubMed
-
- Lee G, Hynes R, Kirschner M. 1984;36:729–740. Temporal and spatial regulation of fibronectin in early Xenopus development. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

