Mitogen activated protein kinase at the nuclear pore complex
- PMID: 20497490
- PMCID: PMC3922677
- DOI: 10.1111/j.1582-4934.2010.01093.x
Mitogen activated protein kinase at the nuclear pore complex
Abstract
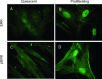
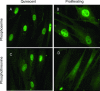
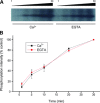
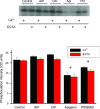
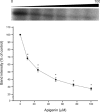
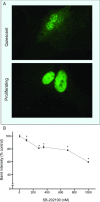
Mitogen activated protein (MAP) kinases control eukaryotic proliferation, and import of kinases into the nucleus through the nuclear pore complex (NPC) can influence gene expression to affect cellular growth, cell viability and homeostatic function. The NPC is a critical regulatory checkpoint for nucleocytoplasmic traffic that regulates gene expression and cell growth, and MAP kinases may be physically associated with the NPC to modulate transport. In the present study, highly enriched NPC fractions were isolated and investigated for associated kinases and/or activity. Endogenous kinase activity was identified within the NPC fraction, which phosphorylated a 30 kD nuclear pore protein. Phosphomodification of this nucleoporin, here termed Nup30, was inhibited by apigenin and PD-98059, two MAP kinase antagonists as well as with SB-202190, a pharmacological blocker of p38. Furthermore, high throughput profiling of enriched NPCs revealed constitutive presence of all members of the MAP kinase family, extracellular regulated kinases (ERK), p38 and Jun N-terminal kinase. The NPC thus contains a spectrum of associated MAP kinases that suggests an intimate role for ERK and p38 in regulation of nuclear pore function.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

Similar articles
-
Insulin activates p38 mitogen-activated protein (MAP) kinase via a MAP kinase kinase (MKK) 3/MKK 6 pathway in vascular smooth muscle cells.Eur J Clin Invest. 2000 Aug;30(8):668-77. doi: 10.1046/j.1365-2362.2000.00671.x. Eur J Clin Invest. 2000. PMID: 10964158
-
Characterization of platelet-derived growth factor-induced p38 mitogen-activated protein kinase activation in vascular smooth muscle cells.Eur J Clin Invest. 2001 Aug;31(8):672-80. doi: 10.1046/j.1365-2362.2001.00865.x. Eur J Clin Invest. 2001. PMID: 11473568
-
Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species.Arterioscler Thromb Vasc Biol. 2000 Apr;20(4):940-8. doi: 10.1161/01.atv.20.4.940. Arterioscler Thromb Vasc Biol. 2000. PMID: 10764657
-
Photodynamic therapy with an endocytically located photosensitizer cause a rapid activation of the mitogen-activated protein kinases extracellular signal-regulated kinase, p38, and c-Jun NH2 terminal kinase with opposing effects on cell survival.Mol Cancer Ther. 2008 Jun;7(6):1740-50. doi: 10.1158/1535-7163.MCT-08-0020. Mol Cancer Ther. 2008. PMID: 18566245
-
Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2.Curr Biol. 1998 Sep 24;8(19):1049-57. doi: 10.1016/s0960-9822(98)70442-7. Curr Biol. 1998. PMID: 9768359 Review.
Cited by
-
Structure, Maintenance, and Regulation of Nuclear Pore Complexes: The Gatekeepers of the Eukaryotic Genome.Cold Spring Harb Perspect Biol. 2022 Mar 1;14(3):a040691. doi: 10.1101/cshperspect.a040691. Cold Spring Harb Perspect Biol. 2022. PMID: 34312247 Free PMC article. Review.
-
Nuclear Membrane Protein SUN5 Is Highly Expressed and Promotes Proliferation and Migration in Colorectal Cancer by Regulating the ERK Pathway.Cancers (Basel). 2022 Oct 31;14(21):5368. doi: 10.3390/cancers14215368. Cancers (Basel). 2022. PMID: 36358787 Free PMC article.
-
Nuclear transport: a switch for the oxidative stress-signaling circuit?J Signal Transduct. 2012;2012:208650. doi: 10.1155/2012/208650. Epub 2011 Oct 15. J Signal Transduct. 2012. PMID: 22028962 Free PMC article.
-
Nuclear pore and nucleocytoplasmic transport impairment in oxidative stress-induced neurodegeneration: relevance to molecular mechanisms in Pathogenesis of Parkinson's and other related neurodegenerative diseases.Mol Neurodegener. 2024 Nov 23;19(1):87. doi: 10.1186/s13024-024-00774-0. Mol Neurodegener. 2024. PMID: 39578912 Free PMC article. Review.
References
-
- Pearson G, Robinson F, Gibson TB, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. - PubMed
-
- Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–8. - PubMed
-
- Jirmanova L, Afanassieff M, Gobert-Gosse S, et al. Differential contributions of ERK and PI3-kinase to the regulation of cyclin D1 expression and to the control of the G1/S transition in mouse embryonic stem cells. Oncogene. 2002;21:5515–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

