5'PPP-RNA induced RIG-I activation inhibits drug-resistant avian H5N1 as well as 1918 and 2009 pandemic influenza virus replication
- PMID: 20492658
- PMCID: PMC2891689
- DOI: 10.1186/1743-422X-7-102
5'PPP-RNA induced RIG-I activation inhibits drug-resistant avian H5N1 as well as 1918 and 2009 pandemic influenza virus replication
Abstract
Background: Emergence of drug-resistant strains of influenza viruses, including avian H5N1 with pandemic potential, 1918 and 2009 A/H1N1 pandemic viruses to currently used antiviral agents, neuraminidase inhibitors and M2 Ion channel blockers, underscores the importance of developing novel antiviral strategies. Activation of innate immune pathogen sensor Retinoic Acid Inducible Gene-I (RIG-I) has recently been shown to induce antiviral state.
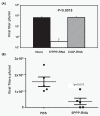
Results: In the present investigation, using real time RT-PCR, immunofluorescence, immunoblot, and plaque assay we show that 5'PPP-containing single stranded RNA (5'PPP-RNA), a ligand for the intracytoplasmic RNA sensor, RIG-I can be used as a prophylactic agent against known drug-resistant avian H5N1 and pandemic influenza viruses. 5'PPP-RNA treatment of human lung epithelial cells inhibited replication of drug-resistant avian H5N1 as well as 1918 and 2009 pandemic influenza viruses in a RIG-I and type 1 interferon dependant manner. Additionally, 5'PPP-RNA treatment also inhibited 2009 H1N1 viral replication in vivo in mice.
Conclusions: Our findings suggest that 5'PPP-RNA mediated activation of RIG-I can suppress replication of influenza viruses irrespective of their genetic make-up, pathogenicity, and drug-sensitivity status.
Figures


Similar articles
-
Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists.J Virol. 2015 Aug;89(15):8011-25. doi: 10.1128/JVI.00845-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018150 Free PMC article.
-
The 3' untranslated regions of influenza genomic sequences are 5'PPP-independent ligands for RIG-I.PLoS One. 2012;7(3):e32661. doi: 10.1371/journal.pone.0032661. Epub 2012 Mar 15. PLoS One. 2012. PMID: 22438882 Free PMC article.
-
Interactions between the influenza A virus RNA polymerase components and retinoic acid-inducible gene I.J Virol. 2014 Sep;88(18):10432-47. doi: 10.1128/JVI.01383-14. Epub 2014 Jun 18. J Virol. 2014. PMID: 24942585 Free PMC article.
-
Influenza viruses resistant to neuraminidase inhibitors.Acta Biochim Pol. 2014;61(3):505-8. Epub 2014 Sep 8. Acta Biochim Pol. 2014. PMID: 25195142 Review.
-
Influenza in the tropics.Curr Opin Infect Dis. 2010 Oct;23(5):415-20. doi: 10.1097/QCO.0b013e32833cc955. Curr Opin Infect Dis. 2010. PMID: 20644472 Review.
Cited by
-
Induction of Interferon-Stimulated Genes Correlates with Reduced Growth of Influenza A Virus in Lungs after RIG-I Agonist Treatment of Ferrets.J Virol. 2022 Aug 24;96(16):e0055922. doi: 10.1128/jvi.00559-22. Epub 2022 Aug 2. J Virol. 2022. PMID: 35916513 Free PMC article.
-
RIG-I activation inhibits HIV replication in macrophages.J Leukoc Biol. 2013 Aug;94(2):337-41. doi: 10.1189/jlb.0313158. Epub 2013 Jun 6. J Leukoc Biol. 2013. PMID: 23744645 Free PMC article.
-
Antiviral Approaches against Influenza Virus.Clin Microbiol Rev. 2023 Mar 23;36(1):e0004022. doi: 10.1128/cmr.00040-22. Epub 2023 Jan 16. Clin Microbiol Rev. 2023. PMID: 36645300 Free PMC article. Review.
-
Critical role of constitutive type I interferon response in bronchial epithelial cell to influenza infection.PLoS One. 2012;7(3):e32947. doi: 10.1371/journal.pone.0032947. Epub 2012 Mar 2. PLoS One. 2012. PMID: 22396801 Free PMC article.
-
Antiviral strategies against influenza virus: towards new therapeutic approaches.Cell Mol Life Sci. 2014 Oct;71(19):3659-83. doi: 10.1007/s00018-014-1615-2. Epub 2014 Apr 4. Cell Mol Life Sci. 2014. PMID: 24699705 Free PMC article. Review.
References
-
- Nicholson KG, McNally T, Silverman M, Simons P, Zambon MC. Influenza-related hospitalizations among young children in Leicestershire. Pediatr Infect Dis J. 2003;22:S228–30. - PubMed
-
- OIE. Update on avian influenza in animals (type H5) 2008. http://www.oie.int/downld/AVIAN%20INFLUENZA/A_AI-Asia.htm Accessed on 02/29/2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

