Lead-induced accumulation of beta-amyloid in the choroid plexus: role of low density lipoprotein receptor protein-1 and protein kinase C
- PMID: 20488202
- PMCID: PMC2934890
- DOI: 10.1016/j.neuro.2010.05.004
Lead-induced accumulation of beta-amyloid in the choroid plexus: role of low density lipoprotein receptor protein-1 and protein kinase C
Abstract
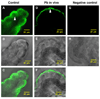
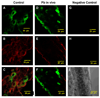
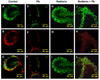
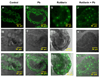
The choroid plexus (CP), constituting the blood-cerebrospinal fluid barrier, has the capacity to remove beta-amyloid (Abeta) from the cerebrospinal fluid. Our previous work indicates that exposure to lead (Pb) results in Abeta accumulation in the CP by decreasing the expression of low density lipoprotein receptor protein-1 (LRP1), a protein involved in the transport and clearance of Abeta. The current study was designed to explore the relationship between Abeta accumulation, protein kinase C (PKC) activity, and LRP1 status in the CP following Pb exposure. Confocal microscopy revealed that LRP1 was primarily localized in the cytosol of the CP in control rats and migrated distinctly towards the apical surface and the microvilli following acute Pb exposure (27 mg Pb/kg, i.p., 24h). Co-immunostaining revealed a co-localization of both PKC-delta and LRP1 in the cytosol of control rats, with a distinct relocalization of both towards the apical membrane following Pb exposure. Preincubation of the tissues with PKC-delta inhibitor rottlerin (2 microM) prior to Pb exposure in vitro, resulted in abolishing the Pb-induced relocalization of LRP1 to the apical surface. Importantly, a significant elevation in intracellular Abeta levels (p<0.01) was observed in the cytosol of the CP following Pb exposure, which was abolished following preincubation with rottlerin. In addition, rottlerin caused a relocalization of Abeta from the cytosol to the nucleus in both Pb-treated and control CP tissues. Finally, co-immunoprecipitation studies revealed a strong protein-protein interaction between LRP1 and PKC-delta in the CP. These studies suggest that Pb exposure disrupts Abeta homeostasis at the CP, owing partly to a Pb-induced relocalization of LRP1 via PKC-delta.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1.Toxicol Appl Pharmacol. 2009 Oct 15;240(2):245-54. doi: 10.1016/j.taap.2009.05.024. Epub 2009 Jun 6. Toxicol Appl Pharmacol. 2009. PMID: 19501112 Free PMC article.
-
Altered clearance of beta-amyloid from the cerebrospinal fluid following subchronic lead exposure in rats: Roles of RAGE and LRP1 in the choroid plexus.J Trace Elem Med Biol. 2020 Apr 8;61:126520. doi: 10.1016/j.jtemb.2020.126520. Online ahead of print. J Trace Elem Med Biol. 2020. PMID: 32325398 Free PMC article.
-
Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice.Neurosci Lett. 2011 Feb 18;490(1):16-20. doi: 10.1016/j.neulet.2010.12.017. Epub 2010 Dec 16. Neurosci Lett. 2011. PMID: 21167913 Free PMC article.
-
Endothelial LRP1 - A Potential Target for the Treatment of Alzheimer's Disease : Theme: Drug Discovery, Development and Delivery in Alzheimer's Disease Guest Editor: Davide Brambilla.Pharm Res. 2017 Dec;34(12):2637-2651. doi: 10.1007/s11095-017-2267-3. Epub 2017 Sep 25. Pharm Res. 2017. PMID: 28948494 Review.
-
Clearance of amyloid-β peptide across the choroid plexus in Alzheimer's disease.Curr Aging Sci. 2010 Dec;3(3):219-29. doi: 10.2174/1874609811003030219. Curr Aging Sci. 2010. PMID: 20735345 Review.
Cited by
-
Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases.Front Cell Neurosci. 2015 Apr 10;9:124. doi: 10.3389/fncel.2015.00124. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25914621 Free PMC article. Review.
-
Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid β-peptide elimination from the brain.J Neurochem. 2010 Dec;115(5):1077-89. doi: 10.1111/j.1471-4159.2010.07002.x. Epub 2010 Oct 5. J Neurochem. 2010. PMID: 20854368 Free PMC article. Review.
-
Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer's disease.J Alzheimers Dis. 2013;33 Suppl 1(0 1):S87-100. doi: 10.3233/JAD-2012-129037. J Alzheimers Dis. 2013. PMID: 22751174 Free PMC article. Review.
-
The role of environmental exposures in neurodegeneration and neurodegenerative diseases.Toxicol Sci. 2011 Dec;124(2):225-50. doi: 10.1093/toxsci/kfr239. Epub 2011 Sep 13. Toxicol Sci. 2011. PMID: 21914720 Free PMC article. Review.
-
Dissecting the Contribution of Vascular Alterations and Aging to Alzheimer's Disease.Mol Neurobiol. 2016 Aug;53(6):3793-3811. doi: 10.1007/s12035-015-9319-7. Epub 2015 Jul 5. Mol Neurobiol. 2016. PMID: 26143259 Review.
References
-
- Liu G, Huang W, Moir RD, Vanderburg CR, Lai B, Peng Z, Tanzi RE, Rogers JT, Huang X. Metal exposure and Alzheimer's pathogenesis. J Struct Biol. 2006;155:45–51. - PubMed
-
- Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol Rev. 2002;54:469–525. - PubMed
-
- Wu J, Basha MR, Zawia NH. The environment, epigenetics and amyloidogenesis. J Mol Neurosci. 2008a;34:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

