Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa
- PMID: 20485566
- PMCID: PMC2869315
- DOI: 10.1371/journal.ppat.1000902
Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa
Abstract
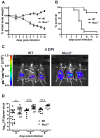
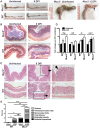
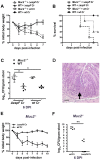
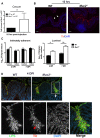
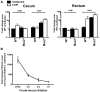
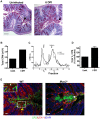
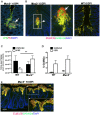
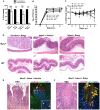
Despite recent advances in our understanding of the pathogenesis of attaching and effacing (A/E) Escherichia coli infections, the mechanisms by which the host defends against these microbes are unclear. The goal of this study was to determine the role of goblet cell-derived Muc2, the major intestinal secretory mucin and primary component of the mucus layer, in host protection against A/E pathogens. To assess the role of Muc2 during A/E bacterial infections, we inoculated Muc2 deficient (Muc2(-/-)) mice with Citrobacter rodentium, a murine A/E pathogen related to diarrheagenic A/E E. coli. Unlike wildtype (WT) mice, infected Muc2(-/-) mice exhibited rapid weight loss and suffered up to 90% mortality. Stool plating demonstrated 10-100 fold greater C. rodentium burdens in Muc2(-/-) vs. WT mice, most of which were found to be loosely adherent to the colonic mucosa. Histology of Muc2(-/-) mice revealed ulceration in the colon amid focal bacterial microcolonies. Metabolic labeling of secreted mucins in the large intestine demonstrated that mucin secretion was markedly increased in WT mice during infection compared to uninfected controls, suggesting that the host uses increased mucin release to flush pathogens from the mucosal surface. Muc2 also impacted host-commensal interactions during infection, as FISH analysis revealed C. rodentium microcolonies contained numerous commensal microbes, which was not observed in WT mice. Orally administered FITC-Dextran and FISH staining showed significantly worsened intestinal barrier disruption in Muc2(-/-) vs. WT mice, with overt pathogen and commensal translocation into the Muc2(-/-) colonic mucosa. Interestingly, commensal depletion enhanced C. rodentium colonization of Muc2(-/-) mice, although colonic pathology was not significantly altered. In conclusion, Muc2 production is critical for host protection during A/E bacterial infections, by limiting overall pathogen and commensal numbers associated with the colonic mucosal surface. Such actions limit tissue damage and translocation of pathogenic and commensal bacteria across the epithelium.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
Stuck to MUC2.Nat Rev Microbiol. 2010 Jul;8(7):463. doi: 10.1038/nrmicro2390. Nat Rev Microbiol. 2010. PMID: 21394951 No abstract available.
Similar articles
-
Formyl peptide receptor 2 orchestrates mucosal protection against Citrobacter rodentium infection.Virulence. 2019 Dec;10(1):610-624. doi: 10.1080/21505594.2019.1635417. Virulence. 2019. PMID: 31234710 Free PMC article.
-
Dietary vitamin D3 deficiency alters intestinal mucosal defense and increases susceptibility to Citrobacter rodentium-induced colitis.Am J Physiol Gastrointest Liver Physiol. 2015 Nov 1;309(9):G730-42. doi: 10.1152/ajpgi.00006.2015. Epub 2015 Sep 3. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26336925 Free PMC article.
-
Goblet Cell Derived RELM-β Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation.PLoS Pathog. 2015 Aug 18;11(8):e1005108. doi: 10.1371/journal.ppat.1005108. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26285214 Free PMC article.
-
The Network of Colonic Host Defense Peptides as an Innate Immune Defense Against Enteropathogenic Bacteria.Front Immunol. 2020 May 20;11:965. doi: 10.3389/fimmu.2020.00965. eCollection 2020. Front Immunol. 2020. PMID: 32508838 Free PMC article. Review.
-
Citrobacter rodentium: infection, inflammation and the microbiota.Nat Rev Microbiol. 2014 Sep;12(9):612-23. doi: 10.1038/nrmicro3315. Epub 2014 Aug 4. Nat Rev Microbiol. 2014. PMID: 25088150 Review.
Cited by
-
Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects.Nutrients. 2022 Sep 16;14(18):3833. doi: 10.3390/nu14183833. Nutrients. 2022. PMID: 36145206 Free PMC article.
-
Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica serovar Typhimurium curli fibrils in the gut.Infect Immun. 2013 Feb;81(2):478-86. doi: 10.1128/IAI.00453-12. Epub 2012 Dec 3. Infect Immun. 2013. PMID: 23208603 Free PMC article.
-
Core 1- and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice.Mucosal Immunol. 2017 Jan;10(1):91-103. doi: 10.1038/mi.2016.45. Epub 2016 May 4. Mucosal Immunol. 2017. PMID: 27143302 Free PMC article.
-
Goblet cells need some stress.J Clin Invest. 2022 Sep 1;132(17):e162030. doi: 10.1172/JCI162030. J Clin Invest. 2022. PMID: 36047499 Free PMC article.
-
L-fucose reduces gut inflammation due to T-regulatory response in Muc2 null mice.PLoS One. 2022 Dec 30;17(12):e0278714. doi: 10.1371/journal.pone.0278714. eCollection 2022. PLoS One. 2022. PMID: 36584066 Free PMC article.
References
-
- Fagundes-Neto U, Andrade JABd. Acute Diarrhea and Malnutrition: Lethality Risk in Hospitalized Infants. J Am Coll Nutr. 1999;18:303–308. - PubMed
-
- Vilchez S, Reyes D, Paniagua M, Bucardo F, Mollby R, et al. Prevalence of diarrhoeagenic Escherichia coli in children from Leon, Nicaragua. J Med Microbiol. 2009;58:630–637. - PubMed
-
- Gonzalez Garcia EA. Animal health and foodborne pathogens: enterohaemorrhagic O157:H7 strains and other pathogenic Escherichia coli virotypes (EPEC, ETEC, EIEC, EHEC). Pol J Vet Sci. 2002;5:103–115. - PubMed
-
- Karch H, Tarr PI, Bielaszewska M. Enterohaemorrhagic Escherichia coli in human medicine. International Journal of Medical Microbiology. 2005;295:405–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

