Managing phenol contents in crop plants by phytochemical farming and breeding-visions and constraints
- PMID: 20479987
- PMCID: PMC2868352
- DOI: 10.3390/ijms11030807
Managing phenol contents in crop plants by phytochemical farming and breeding-visions and constraints
Abstract
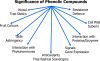
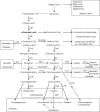
Two main fields of interest form the background of actual demand for optimized levels of phenolic compounds in crop plants. These are human health and plant resistance to pathogens and to biotic and abiotic stress factors. A survey of agricultural technologies influencing the biosynthesis and accumulation of phenolic compounds in crop plants is presented, including observations on the effects of light, temperature, mineral nutrition, water management, grafting, elevated atmospheric CO(2), growth and differentiation of the plant and application of elicitors, stimulating agents and plant activators. The underlying mechanisms are discussed with respect to carbohydrate availability, trade-offs to competing demands as well as to regulatory elements. Outlines are given for genetic engineering and plant breeding. Constraints and possible physiological feedbacks are considered for successful and sustainable application of agricultural techniques with respect to management of plant phenol profiles and concentrations.
Keywords: agricultural technology; apple; elicitor; flavonoids; grapevine; lettuce; phenylpropanoids; strawberry; stress; tomato.
Figures


Similar articles
-
Enhancement of Plant Productivity in the Post-Genomics Era.Curr Genomics. 2016 Aug;17(4):295-6. doi: 10.2174/138920291704160607182507. Curr Genomics. 2016. PMID: 27499678 Free PMC article.
-
Evolution and Application of Genome Editing Techniques for Achieving Food and Nutritional Security.Int J Mol Sci. 2021 May 25;22(11):5585. doi: 10.3390/ijms22115585. Int J Mol Sci. 2021. PMID: 34070430 Free PMC article. Review.
-
Transgenic Breeding Approaches for Improving Abiotic Stress Tolerance: Recent Progress and Future Perspectives.Int J Mol Sci. 2020 Apr 13;21(8):2695. doi: 10.3390/ijms21082695. Int J Mol Sci. 2020. PMID: 32295026 Free PMC article. Review.
-
Health-promoting phytochemicals of Italian common wheat varieties grown under low-input agricultural management.J Sci Food Agric. 2012 Nov;92(14):2800-10. doi: 10.1002/jsfa.5590. Epub 2012 Jan 25. J Sci Food Agric. 2012. PMID: 22278616
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
Cited by
-
Unlike quercetin glycosides, cyanidin glycoside in red leaf lettuce responds more sensitively to increasing low radiation intensity before than after head formation has started.J Agric Food Chem. 2014 Jul 23;62(29):6911-7. doi: 10.1021/jf404782n. Epub 2014 Jan 9. J Agric Food Chem. 2014. PMID: 24382136 Free PMC article.
-
Morphological and Biochemical Diversity of Shallot Landraces Preserved Along the Croatian Coast.Front Plant Sci. 2018 Dec 3;9:1749. doi: 10.3389/fpls.2018.01749. eCollection 2018. Front Plant Sci. 2018. PMID: 30559753 Free PMC article.
-
Abscisic acid induced changes in production of primary and secondary metabolites, photosynthetic capacity, antioxidant capability, antioxidant enzymes and lipoxygenase inhibitory activity of Orthosiphon stamineus Benth.Molecules. 2013 Jul 5;18(7):7957-76. doi: 10.3390/molecules18077957. Molecules. 2013. PMID: 23884129 Free PMC article.
-
Growth and Biochemical Composition of Microgreens Grown in Different Formulated Soilless Media.Plants (Basel). 2022 Dec 15;11(24):3546. doi: 10.3390/plants11243546. Plants (Basel). 2022. PMID: 36559657 Free PMC article.
-
Bioactivity of Nonedible Parts of Punica granatum L.: A Potential Source of Functional Ingredients.Int J Food Sci. 2013;2013:602312. doi: 10.1155/2013/602312. Epub 2013 Jul 8. Int J Food Sci. 2013. PMID: 26904607 Free PMC article.
References
-
- Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet. 1993;342:1007–1011. - PubMed
-
- Watzl B, Leitzmann C. Bioaktive Substanzen in Lebensmitteln. 3rd ed. Hippokrates Verlag; Tubingen, Germany: 2005.
-
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. - PubMed
-
- Gohil K, Packer L. Bioflavonoid-rich botanical extracts show antioxidant and gene regulatory activity. Ann. NY Acad. Sci. 2002;957:70–77. - PubMed
-
- Bensath A, Ruysnyak T, Szent-Györgyi A. Vitamin nature of flavones. Nature. 1936;138:789–793.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

