Redundant function of cmaA2 and mmaA2 in Mycobacterium tuberculosis cis cyclopropanation of oxygenated mycolates
- PMID: 20472794
- PMCID: PMC2897352
- DOI: 10.1128/JB.00312-10
Redundant function of cmaA2 and mmaA2 in Mycobacterium tuberculosis cis cyclopropanation of oxygenated mycolates
Abstract
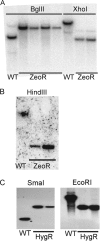
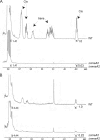
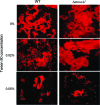
The Mycobacterium tuberculosis cell envelope contains a wide variety of lipids and glycolipids, including mycolic acids, long-chain branched fatty acids that are decorated by cyclopropane rings. Genetic analysis of the mycolate methyltransferase family has been a powerful approach to assign functions to each of these enzymes but has failed to reveal the origin of cis cyclopropanation of the oxygenated mycolates. Here we examine potential redundancy between mycolic acid methyltransferases by generating and analyzing M. tuberculosis strains lacking mmaA2 and cmaA2, mmaA2 and cmaA1, or mmaA1 alone. M. tuberculosis lacking both cmaA2 and mmaA2 cannot cis cyclopropanate methoxymycolates or ketomycolates, phenotypes not shared by the mmaA2 and cmaA2 single mutants. In contrast, a combined loss of cmaA1 and mmaA2 had no effect on mycolic acid modification compared to results with a loss of mmaA2 alone. Deletion of mmaA1 from M. tuberculosis abolishes trans cyclopropanation without accumulation of trans-unsaturated oxygenated mycolates, placing MmaA1 in the biosynthetic pathway for trans-cyclopropanated oxygenated mycolates before CmaA2. These results define new functions for the mycolic acid methyltransferases of M. tuberculosis and indicate a substantial redundancy of function for MmaA2 and CmaA2, the latter of which can function as both a cis and trans cyclopropane synthase for the oxygenated mycolates.
Figures



Similar articles
-
The mmaA2 gene of Mycobacterium tuberculosis encodes the distal cyclopropane synthase of the alpha-mycolic acid.J Biol Chem. 2003 Mar 7;278(10):7844-9. doi: 10.1074/jbc.M212458200. Epub 2002 Dec 26. J Biol Chem. 2003. PMID: 12502719
-
Trans-cyclopropanation of mycolic acids on trehalose dimycolate suppresses Mycobacterium tuberculosis -induced inflammation and virulence.J Clin Invest. 2006 Jun;116(6):1660-7. doi: 10.1172/JCI27335. J Clin Invest. 2006. PMID: 16741578 Free PMC article.
-
The Mycobacterium tuberculosis cmaA2 gene encodes a mycolic acid trans-cyclopropane synthetase.J Biol Chem. 2001 Jan 19;276(3):2228-33. doi: 10.1074/jbc.C000652200. Epub 2000 Nov 22. J Biol Chem. 2001. PMID: 11092877
-
Mycolic acids: deciphering and targeting the Achilles' heel of the tubercle bacillus.Mol Microbiol. 2015 Oct;98(1):7-16. doi: 10.1111/mmi.13101. Epub 2015 Jul 30. Mol Microbiol. 2015. PMID: 26135034 Free PMC article. Review.
-
Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis.Clin Microbiol Rev. 2005 Jan;18(1):81-101. doi: 10.1128/CMR.18.1.81-101.2005. Clin Microbiol Rev. 2005. PMID: 15653820 Free PMC article. Review.
Cited by
-
Cyclopropane-Containing Fatty Acids from the Marine Bacterium Labrenzia sp. 011 with Antimicrobial and GPR84 Activity.Mar Drugs. 2018 Oct 8;16(10):369. doi: 10.3390/md16100369. Mar Drugs. 2018. PMID: 30297608 Free PMC article.
-
MapB Protein is the Essential Methionine Aminopeptidase in Mycobacterium tuberculosis.Cells. 2019 Apr 28;8(5):393. doi: 10.3390/cells8050393. Cells. 2019. PMID: 31035386 Free PMC article.
-
Rv0132c of Mycobacterium tuberculosis encodes a coenzyme F420-dependent hydroxymycolic acid dehydrogenase.PLoS One. 2013 Dec 11;8(12):e81985. doi: 10.1371/journal.pone.0081985. eCollection 2013. PLoS One. 2013. PMID: 24349169 Free PMC article.
-
Phospholipid homeostasis, membrane tenacity and survival of Mtb in lipid rich conditions is determined by MmpL11 function.Sci Rep. 2018 May 29;8(1):8317. doi: 10.1038/s41598-018-26710-z. Sci Rep. 2018. PMID: 29844505 Free PMC article.
-
Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to β-lactams.J Bacteriol. 2014 Apr;196(7):1394-402. doi: 10.1128/JB.01396-13. Epub 2014 Jan 24. J Bacteriol. 2014. PMID: 24464457 Free PMC article.
References
-
- Alahari, A., L. Alibaud, X. Trivelli, R. Gupta, G. Lamichhane, R. C. Reynolds, W. R. Bishai, Y. Guerardel, and L. Kremer. 2009. Mycolic acid methyltransferase, MmaA4, is necessary for thiacetazone susceptibility in Mycobacterium tuberculosis. Mol. Microbiol. 71:1263-1277. - PubMed
-
- Barry, C. E., III, R. E. Lee, K. Mdluli, A. E. Sampson, B. G. Schroeder, R. A. Slayden, and Y. Yuan. 1998. Mycolic acids: structure, biosynthesis and physiological functions. Prog. Lipid Res. 37:143-179. - PubMed
-
- Bhatt, A., N. Fujiwara, K. Bhatt, S. S. Gurcha, L. Kremer, B. Chen, J. Chan, S. A. Porcelli, K. Kobayashi, G. S. Besra, and W. R. Jacobs, Jr. 2007. Deletion of kasB in Mycobacterium tuberculosis causes loss of acid-fastness and subclinical latent tuberculosis in immunocompetent mice. Proc. Natl. Acad. Sci. U. S. A. 104:5157-5162. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

